Saguinus nigricollis (Spix, 1823)
|
publication ID |
https://doi.org/ 10.5281/zenodo.5730714 |
|
DOI |
https://doi.org/10.5281/zenodo.5730854 |
|
persistent identifier |
https://treatment.plazi.org/id/DF668780-FFC7-FFD6-FFDB-FE496A14E6AF |
|
treatment provided by |
Conny |
|
scientific name |
Saguinus nigricollis |
| status |
|
24 View On .
Black-mantled Tamarin
Saguinus nigricollis View in CoL
French: Tamarin rouge et noir / German: Schwarzriickentamarin / Spanish: Tamarin de dorso negro Other common names: Black Mantle Tamarin; Graells's Black-mantled/Graells’s Tamarin (graellsi), Hernandez-Camacho's Black-mantled Tamarin (hernandezi), Spix's Black-mantled Tamarin (nigricollis)
Taxonomy. Midas nigricollis Spix, 1823 View in CoL ,
Brazil, Amazonas, near Sao Paulo de OIivenca, north bank of the Rio Solimoes .
The subspecies graellsi has been considered a distinct species by some, based on its supposed sympatry with the subspecies nigricollis north of the Rio Putumayo in Colombia (area of Puerto Asis and Puerto Leguizamo). A subsequent reassessment indicated that the specimens judged to be nigricollis were in fact dull-colored S. fuscus . A recent molecular genetic study indicated that graellsi and nigricollis were inseparable as species. Three subspecies recognized.
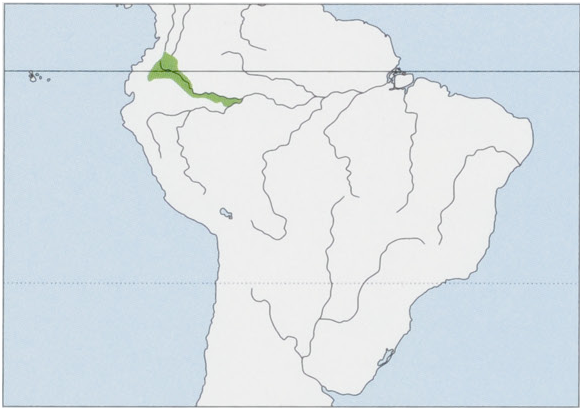
Subspecies and Distribution. S. n. mgricollis Spix, 1823 — Brazilian Amazon, N Peru, and possibly SE Colombia, between the Rio Ica— Putumayo and the rios Solimoes-Amazonas and Napo, W as far the seasonally flooded forest varzea along the Tamboryacu. S. n. graellsiJiménez de la Espada, 1870 — S Colombia, NE Ecuador, and N Peru, S of the upper Rio Caqueta (W from the mouth of the Rio Yari) in Colombia, S to both sides of the upper Putumayo as far as the N (left) bank of the Rio Napo, extending E between the Napo and Putumayo as far as the seasonally flooded forest along the Tamboryacu. S. mn. hernandezi Hershkovitz, 1982 — S Colombia, Meta Department, between the rios Caqueta, Caguan, and Orteguaza and the base of the Cordillera Oriental to the Rio Guayabero. View Figure
Descriptive notes. Head-body 21-25 cm, tail 31-35 cm; weight 420-500 g. In “Spix’s Black-mantled Tamarin” (S. n. nigricollis ), the head, neck, mantle, and forelimbs are black, grading into red or brownish-red on the hindparts. The forehead, crown, throat, and neck are blackish brown. Sides of the crown are brown. Facial skin is black, with short gray hairs around the mouth and nostrils. The base of the back and upper surface ofthe tail are marbled or striated reddish and blackish. Rump and legs and the underneath of the tail are mostly or almost entirely reddish or mahogany. The rest of the tail is blackish. The neck, chest, and belly are blackish, often mixed with red or mahogany color. External genitalia are mostly or entirely pigmented and sparsely covered with brown or reddish hairs. In “Graells’s Black-mantled Tamarin” (S. n. graellsi), the head, throat, mantle, and forelimbs are blackish to blackish brown, finely ticked with gray or buffy. There are pale brown patches on the sides of the head and cheeks, which are distinctive in the adults. The mantle is squared-off rather than tapering asit is in Spix’s Black-mantled Tamarin; it does not extend to the middle and lower back, which are mainly buffy agouti along with sides of the body, thighs,legs, and proximate dorsal part of the tail. The tail is otherwise blackish. The neck, chest, and belly vary from buffy agouti to blackish. Facial skin is black. Hairs around the mouth and sides of the nostrils are gray. The skin of external genitalia is black. The forehead and crown are blackish. In “Hernandez-Camacho’s Black-mantled Tamarin” (S. n. hernandezi), the nape, mantle, and throat are blackish. It differs from the other two subspecies in that the mantle tapers behind to the middle of the back and extends as a stripe down the back and onto the tail. Sides of the lower one-half of the back are orange-agouti and have a marbled appearance. The neck, chest, arms, legs, rump, and sides of the body are dominantly orange-agouti. The base of the hairs on the neck and throat are whitish. The belly is mixed orange and blackish. Sides of the crown and face have contrastingly paler hairs. Sides of the lower back are mixed blackish and orange. Sides and ventral surface of the first 5-10 cm of the tail are orange-agouti, but the remainder is black. Facial skin is moderately pigmented. The orange-agouti coloration distinguishes this subspecies, being replaced by buffy agouti in Graells’s Black-mantled Tamarin and reddish or mahogany in Spix’s Black-mantled Tamarin. Hernandez-Camacho’s Blackmantled Tamarin is like Spix’s Black-mantled Tamarin in its dark, tapered mantle, but it also resembles Graells’s Black-mantled Tamarin in its shorter mantle and finely banded, agouti hindlimbs.
Habitat. Information on the Black-mantled Tamarin in the wild comes largely from three field studies: one in the Rio Peneya Basin, Colombia, by K. Izawa (Hernandez-Camacho’s Black-mantled Tamarin); the second in the Cuyabeno Reserve, Ecuador, by S. de la Torre and colleagues (Graells’s Black-mantled Tamarin); and the third on the south bank of the Rio Guayabero in Tinigua National Natural Park, La Macarena, Colombia, by N. Vargas Tovar (Hernandez-Camacho’s Black-mantled Tamarin). Spix’s Black-mantled Tamarin has not been studied in the wild. Black-mantled Tamarins forage and travel mostly in the dense growth of the forest understory at heights of up to 10 m above the ground. In the forest of the Rio Peneya, they used lowerstrata of the forest, mostly 2-10 m from the ground, and they sometimes foraged on the ground. In Ecuador, group home ranges include white-water flooded forest dominated by the palm Mauritia flexuosa, Arecaceae (locally referred to as “cananguchal”), black-water flooded forest (igapo), and seasonally flooded white-water forest (varzea), but they are mainly in terra firma forest. Black-mantled Tamarins forage for animal prey in patches of dense vegetation in disturbed or high secondary forest patches and edge habitat. Rainfall is high in Cuyabeno (3000 mm/year, with more than 250 mm/month during the rainy season), and forest productivity is high. The rainy season is from the middle of March through August, and the dry season is from September through early March. In the extreme north ofits distribution, the Black-mantled Tamarin can be found in an area of highly seasonal forest on poor soils (Tinigua National Natural Park). Annual rainfall is ¢.2300 mm, but with a marked dry season with less than 100 mm/month in December—February. In Tinigua, Black-mantled Tamarins spent more than one-third of their time in shrubby secondary growth (no higher than 5 m), which is locally referred to as “arrabal,” and frequently burned by nearby villagers. They spent much of their time (34%) in edge habitat, bordering the river, small lakes, and pastures, and also used tall forest (up to 20 m in height) and palm forest of Syagrus and Attalea , Arecaceae (up to 12 m in height and also severely affected by fire). They spent just over one-half of their time at heights ofless than 10 m above the ground, and nearly onehalf of their time foraging on animal prey was at heights up to 5 m. They used palm trees and vine tangles as sleeping sites.
Food and Feeding. At Cuyabeno, Graells’s Black-mantled Tamarins eat fruits, especially from Pouteria cf. baehniana ( Sapotaceae ) and Protium cf. aracouchini ( Burseraceae ). Other members of these genera were also important in the diet, but the species were not identified. Fruits of Pseudolmedia laevis ( Moraceae ) and Eschweilera cf. coriacea ( Lecythidaceae ) were also important. They eat readily available gums from the bark of Inga marginata, I. ruiziana ( Fabaceae ), and Spondias mombin ( Anacardiaceae ), and flowers and nectar from Araceae , possibly Anthurium . Small prey include grasshoppers ( Orthoptera ), ants ( Hymenoptera ), butterflies ( Lepidoptera ), termites (Isoptera), and beetles ( Coleoptera ). Hernandez-Camacho’s Black-mantled Tamarins in the forests of the Rio Peneya eatfruits, flowers, and also, opportunistically, gum exuded from Inga trunks and Parkia oppositifolia ( Fabaceae ) seed pods. Insects eaten include Orthoptera (particularly the families Tettigoniidae and Acrididae ), Dermaptera, Hemiptera , Coleoptera, Odonata, and Ephemeroptera. The large (6-8 cm long) grasshoppers that they particularly prefer are caught with both hands and then pushed down onto a branch; they eat the head first and then pull out and eat the entrails. They pull off the wings, gnaw at their bases, and then drop them. The body and all or parts of the legs are eaten, exceptfor the thicker, often spiny, hindlegs. Fruits are mainly eaten from trees in the lower canopy and particularly include Leonia cymosa ( Violaceae ) in October, Pourouma lawrancei ( Urticaceae ) in December—January, along with Pseudolmedia laevis, Protium sagotianum ( Burseraceae ), Coussapoa mutisii ( Urticaceae ), and Helicostylis tomentosa (Moraceae) . Black-mantled Tamarins in Tinigua National Natural Park eat fruits of Protium , Pourouma , Cecropia , Ficus , and various species of Melastomataceae , including two species of Miconia in the early wet season. Animal prey include insects (especially Orthoptera in the families Romaleidae , Acrididae , and Tettigoniidae , and lepidopteran larvae) and lizards. They also eat flowers from a species of Ochroma (Bombacaceae) .
Breeding. At Cuyabeno, births of Black-mantled Tamarins are concentrated in January (toward the end of the dry season), and some groups also have infants in June (middle of the wet season). Interbirth interval is about five months. This bimodality is believed to reflect the richness of the forests there, and resource-dependent flexibility on the part of the tamarins—breeding a second time when conditions are favorable. In the Rio Peneya Valley, births were concentrated in the wet season in December—February, but as at Cuyabeno, there was a second smaller birth peak when some groups produced twins in June.
Activity patterns. In the rich forests of Cuyabeno, activity budgets of one group of Black-mantled Tamarins during the dry season and wet season were as follows. In the dry season, 34-8% of the time was spent foraging for animal prey, 27-2% resting, 21% traveling, and 17% feeding. In the rainy season, they foraged for animal prey (38-4%), rested (29-8%), and traveled a bit more (25:6%) and spentless time feeding (6-2%). Differences were perhaps associated with more abundant but more dispersed plant food sources in the wet season. In the dry season, food sources were more concentrated (patchy) and less abundant. In Tinigua, in the far north of the species’ distribution, Black-mantled Tamarins are active longer during the day in the dry season than in the wet season. In the dry season, they initiate their daily activities at 04:00-04:30 h and return to their sleeping site at 18:45-19:15 h. In the wet season, they become active later (05:00-05:30 h) and retire earlier (16:00-16:30 h). In general, the typical length of daily activity for tamarins is 10-4-11-6 hours, but Black-mantled Tamarins at Tinigua are active on average 14-6 hours/day, probably because of the very high midday temperatures and food scarcity, particularly in the dry season. They rest for unusually long periods at midday and have two pronounced peaks of foraging on animal prey and fruit, one in the early morning and anotherlate in the afternoon.
Movements, Home range and Social organization. Black-mantled Tamarins form groups of up to eleven individuals. Ten groups monitored in the Rio Peneya Basin averaged 6-3 ind/group (including infants), three with two adult males and three with two adult females. A group of eight had two adult males and two adult females. In Cuyabeno, ten groups varied in size from a breeding pair to nine individuals (average of five). All but one of the groups had just one adult pair and its offspring. One group had two adult males. Groups at the Rio Peneya had home ranges of 42-56 ha, with considerable overlap (as much as 80%). A group of 7-9 Graells’s Black-mantled Tamarins in the Cuyabeno Reserve occupied a home range of 56-2 ha in the dry season and 41-7 ha in the wet season, and their home range also overlapped extensively (more than 80%) with other groups. Temporary formations of large groups were recorded at all three study sites—the result of 2-3 neighboring groups mingling for sometimes an hour or more with little or no evident antagonism. Densities were 10-13 ind/km*at the Rio Peneya and 22-33 ind/km?*at Cuyabeno. Densities of Spix’s Black-mantled Tamarin along the Rio Pureté, Colombia, were 4-15 ind/km?. Surveys along the Rio Ampiyacu, Peru, found 3-2 groups/km? or 19-2 ind/km?®. Barred forest-falcons (Micrastur ruficollis) are known to prey on Black-mantled Tamarins, and other potential predators include harpy eagles (Harpia harpyja), Tayra (Eira barbara), and Ocelot (Leopardus pardalis).
Status and Conservation. CITES Appendix II. Classified as Least Concern on The [UCN Red List. Recent surveys in Ecuador and Peru, however, have indicated that distribution of Graells’s Black-mantled Tamarin is much smaller than had been supposed, and it is listed as Near Threatened because the region where it occurs has one of the highestrates of deforestation in the western Amazon. Spix’s Black-mantled Tamarin is regarded as common in Peru, and although it was heavily exploited for export for biomedical research in the 1960s and 1970s, it is common in Colombia. Its status in Brazil is unknown. In Colombia, Spix’s Black-mantled Tamarin probably occurs in Amacayacu National Natural Park and possibly La Paya National Natural Park. There are no protected areas in the Peruvian part the distribution of Spix’s Black-mantled Tamarin. In Ecuador, Graells’s Black-mantled Tamarin occurs in the Limoncocha Biological Reserve on the north bank of the Rio Napo, Cuyabeno Reserve, and Wildsumaco Wildlife Sanctuary. Sangay National Park and Cayambé-Coca Faunal Ecological Reserve are within its distribution, but its presence in these protected areas has not been verified. Hernandez-Camacho’s Black-mantled Tamarin has a very small distribution, restricted to Colombia, but no information is available regarding its conservation status. North-east of the headwaters of the Rio Caguan,it occurs in dry forests of the Tinigua National Natural Park. It is not known if it occurs in the Cordillera de los Picachos National Natural Park to the north of Tinigua, or on the west bank of the Rio Guayabero.
Bibliography. Chase & Cooper (1969), Defler (1994b, 2003b, 2004), Freese, Freese & Castro (1977), Freese, Heltne et al. (1982), Hernandez-Camacho & Cooper (1976), Hernandez-Camacho & Defler (1989), Hershkovitz (1977, 1982), Izawa (1975, 1976, 1978a), Snowdon & Soini (1988), Soini et al. (1989), de la Torre (1996, 2000), de la Torre, Campos & de Vries (1995), de la Torre, Utreras & Campos (1995), Vanderhoff & Nillson (2010), Vargas (1994).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.