Alpheus thompsoni Anker, 2022
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5105.3.5 |
|
publication LSID |
lsid:zoobank.org:pub:0055F495-69B0-45A3-8BCD-128A569E9079 |
|
DOI |
https://doi.org/10.5281/zenodo.6343544 |
|
persistent identifier |
https://treatment.plazi.org/id/9F69E057-FFE9-6769-249E-02A1FEDB3401 |
|
treatment provided by |
Plazi |
|
scientific name |
Alpheus thompsoni Anker, 2022 |
| status |
|
( Figs. 6–8 View FIGURE 6 View FIGURE 7 View FIGURE 8 ; 9A View FIGURE 9 )
Alpheus thompsoni Anker 2022: 274 View Cited Treatment , figs. 1–3, 8A, B.
Alpheus ochrostriatus .— Karplus et al. 1981: 6, fig. 2F (black-and-white photograph); Debelius 1997: 203 (colour photograph); Debelius 2001: 150 (part.), 1 colour photograph (yellow morph); Randall et al. 2003: 512, fig. 8 (colour photograph); Randall 2005: 513; Poupin 2010: 33; Karplus & Thompson 2011: 591, fig. 4.4.10-B (black-and-white photograph) [nomen nudum].
Alpheus “ ochrostriatus ”. View in CoL — Anker 2000: 3, fig. 2 (colour photograph); Jaafar & Randall 2009: 29, pl. 2A (colour photograph) [nomen nudum].
Alpheus sp. ‘ ochrostriata ’.— Kuiter & Debelius 2009: 151, 3 colour photographs [nomen nudum].
Alpheus djiboutensis View in CoL .— Yu et al. 1996: 35, figs. 19, 20 (colour photographs) [not A. djiboutensis De Man, 1909 View in CoL ].
Alpheus sp. 4 .— Minemizu 2013: 102, colour photograph.
Material examined. 1 male (cl 10.0 mm), FLMNH UF 37002 , Saudi Arabia, Red Sea , Farasan Islands, Zahrat Durakah, 16°50’09.2”N, 42°18’22.7”E, fringing reef slope around sandy island, depth 2–6 m, leg. A. Anker, P. Norby, G. Paulay, 11.03.2013 [fcn BDJRS-2689]. GoogleMaps
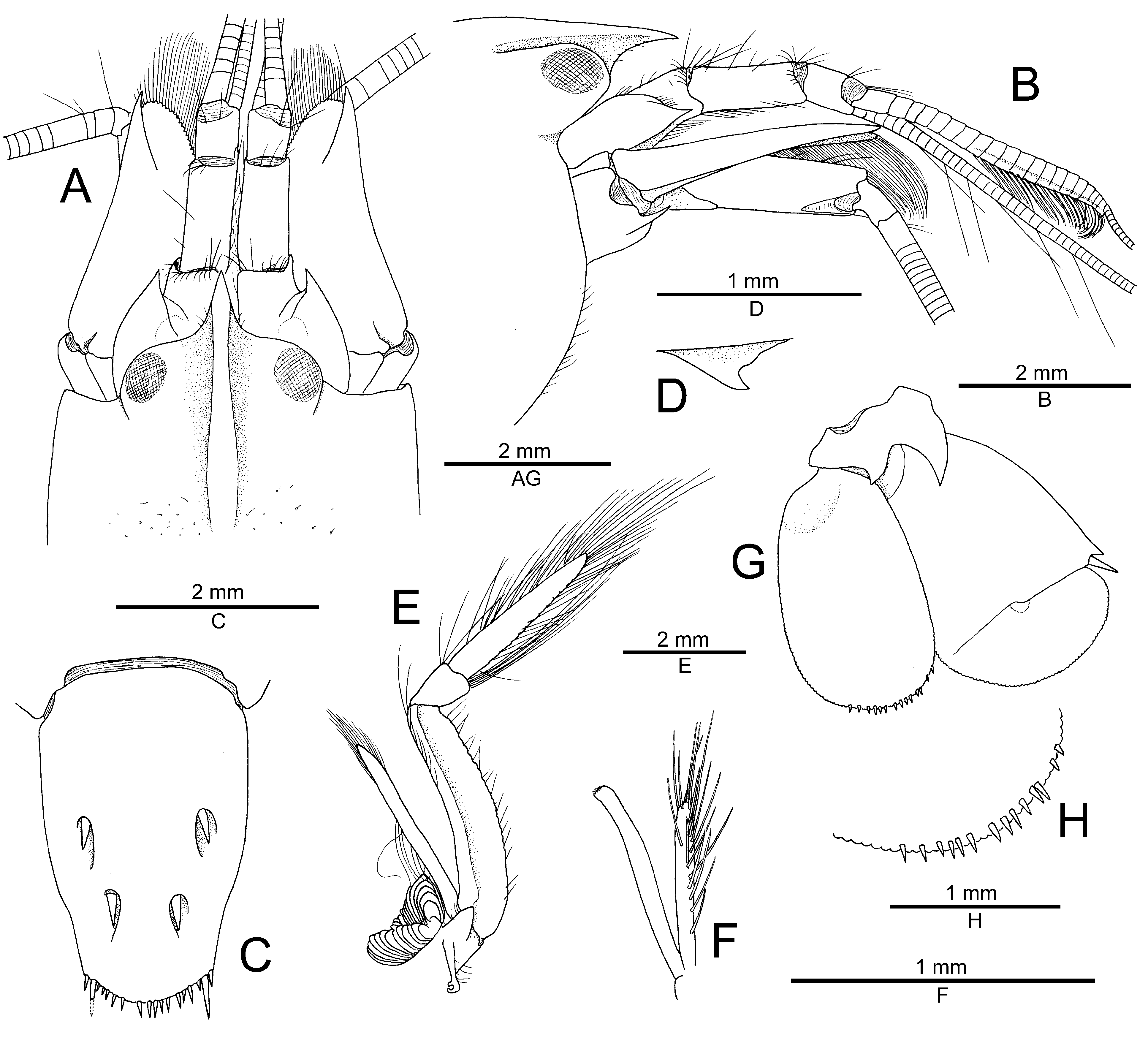
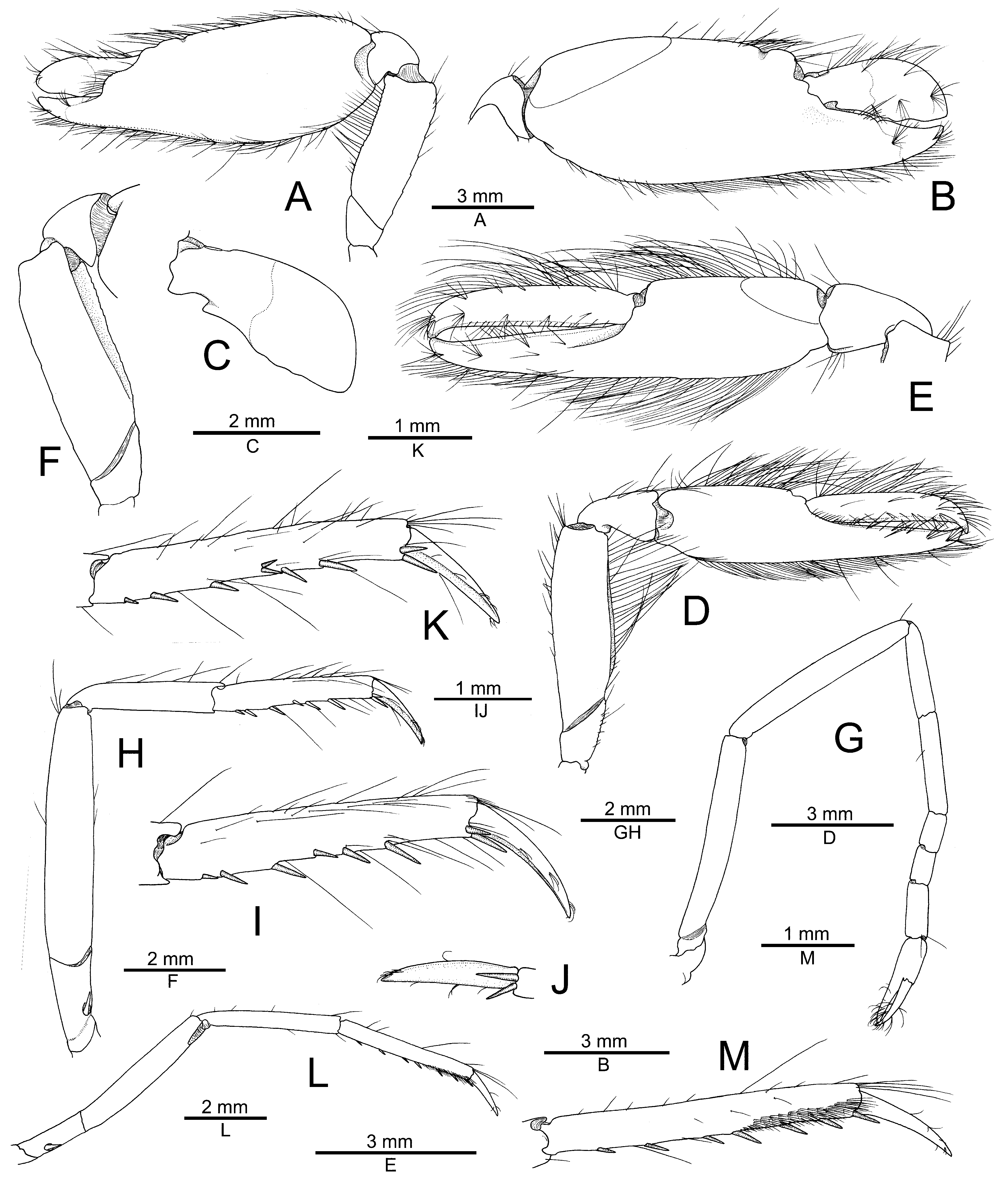
Description. See Anker (2022). Complementary illustrations of the general morphology of the Red Sea male are provided in Figs. 6 View FIGURE 6 , 7 View FIGURE 7 . The variation in the antennal scaphocerite and major and minor chelipeds are discussed below.
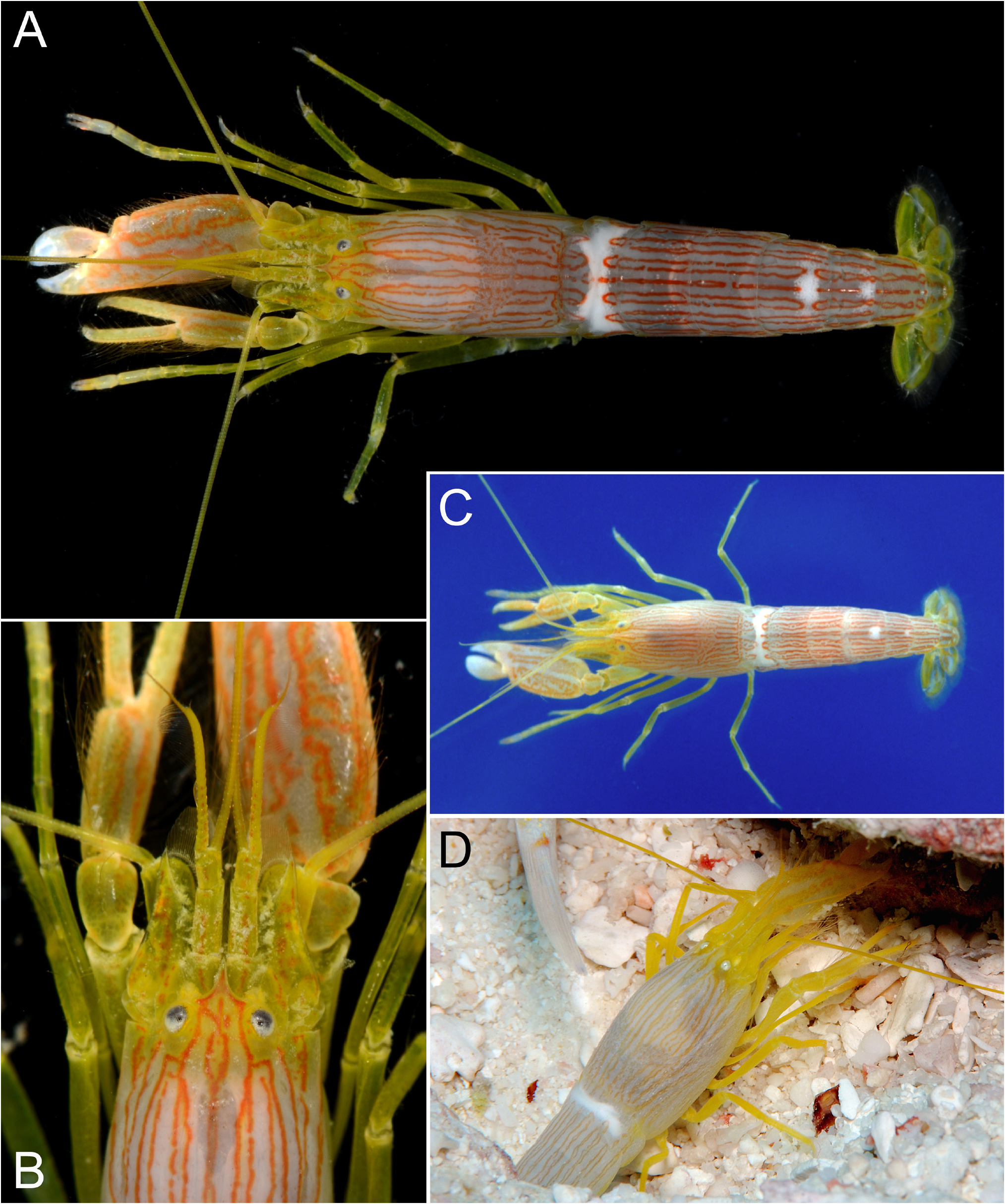
Colour pattern. Anker (2022) described in detail the colour pattern of the female holotype of A. thompsoni from Madang, Papua New Guinea. This colour pattern is also present in shrimps from numerous other localities in Papua New Guinea and Indonesia (e.g. Kuiter & Debelius 2009: Flores and Bali; Fig. 9A View FIGURE 9 , Sulawesi). In the male from Saudi Arabia ( Fig. 8A, B View FIGURE 8 ), the red parallel-running longitudinal bands are simple, i.e. they form simple, elongate, red strings closed at both ends, which is in marked contrast to the more complex bands of the holotype of A. thompsoni (cf. Anker 2022: fig. 3A, B, 8A, B), in which they are broader and filled with a wealth of finer anastomosing bands and streaks (also visible in Fig. 9A View FIGURE 9 ). See also discussion below.
Distribution. Indo-West Pacific from the Red Sea to southern Japan, Indonesia and New Caledonia ( Karplus et al. 1981; Debelius 1997, 2001; Anker 2000, 2022; Randall 2005; Kuiter & Debelius 2009; Poupin 2010; present study).
Ecology. Coral reefs and adjacent reef flats; associated with several species of gobiid fishes, including Amblyeleotris wheeleri ( Polunin & Lubbock, 1977) , A. steinitzi (Klausewitz, 1974) , A. guttata (Fowler, 1938) , A. fasciata (Herre, 1953) , Ctenogobiops pomastictus Polunin & Lubbock, 1977 , and C. tangaroai Lubbock & Polunin, 1977 ( Karplus et al. 1981; Randall et al. 1998, 2003, 2007; Anker 2000, 2022; Debelius 2001; Randall 2005; Kuiter & Debelius 2009; Jaafar & Randall 2009; Karplus & Thompson 2011).
Remarks. The present complete male specimen from Saudi Arabia was initially considered to belong to an undescribed species closely related to A. thompsoni due to some discrepancies in the colour pattern (see above) and a small difference in the shape of the scaphocerite (see below). Most shrimps identified as “ A. ochrostriatus ” or “ Alpheus sp. ” in the published or unpublished colour photographs analysed by the author could be classified as either the species-diagnostic type A pattern, with broader red bands containing fine, often anastomosing lines ( Fig. 9A View FIGURE 9 ; see also Anker 2022: fig. 3), or the type B pattern, with narrower red bands not filled with fine lines ( Fig. 8A, B, D View FIGURE 8 ). It is important to mention that the holotype female at cl 13.3 mm is only slightly larger than the present male at cl 10.0 mm; thus, these two colour pattern types are comparable since they are present in adult individuals. However, after a more detailed analysis and inclusion of more photographic material, the author came to the conclusion that the type A and type B patterns are part of the same general colour pattern of A. thompsoni . The most pivotal observation for this conclusion was an in situ colour photograph in Kuiter & Debelius (2009: 151, upper photograph), showing two individuals cohabiting the same burrow, a larger female at the burrow entrance apparently (slightly out of focus) with the type A pattern and a smaller male in front of her clearly with the type B pattern. In addition, an indication of an intermediate state between the type A and type B pattern can be seen on the pleon of the specimen from southern Taiwan ( Fig. 8C View FIGURE 8 ; same photograph as in Yu et al. 1996: fig. 19).
Differences in the proportions of the major and minor chelipeds between males and females are expected. As is the case of many other species of the A. brevirostris group, both chelipeds of the Saudi Arabian male of A. thompsoni ( Fig. 7A, B, E View FIGURE 7 ) are larger and stronger than their homologs in the female holotype from Papua New Guinea ( Anker 2022: fig. 2). The male minor chela is also lacking balaeniceps ridges and setae ( Fig. 7D, E View FIGURE 7 ), thereby confirming their absence in both sexes in A. thompsoni . The ventromesial margin of the merus of both chelipeds is unarmed in the present male ( Fig. 7A, D, F View FIGURE 7 ) and also has a reduced armature, with only one spiniform seta present on the minor cheliped merus, in the holotype female ( Anker 2022: fig. 2C, F). The presence of spiniform setae on the cheliped merus is usually a consistent character, although their number may be variable; in addition, they break off easily or may be missing entirely in regenerated chelipeds. The relative proportions of the carpal subarticles of the second pereiopod is only slightly different, with the first subarticle being slightly than the second in the holotype female ( Anker 2022: fig. 1F) vs. the two being almost equal in length in the present male ( Fig. 7 View FIGURE 7 ).
The only remaining significant difference between the female holotype from Papua New Guinea and the male from Saudi Arabia lies in the shape of the distal region of the antennal scaphocerite. In the female, the blade extends slightly beyond the distolateral tooth ( Anker 2022: fig. 1A), whereas in the male, it is noticeably shorter than the distolateral tooth ( Fig. 6A View FIGURE 6 ). This difference can be explained either by the somewhat worn distolateral tooth of the holotype (which normally would be slightly longer) or by intraspecific variability. With only two specimens of A. thompsoni available, it is difficult to assess the variation observed in some morphological characters, as well as in the colour pattern. Although the involvement of a second species cannot be excluded at this stage, it seems to be less likely based on the presently available data. The planned DNA analyses of the entire A. djeddensis — A. djiboutensis group should eventually resolve this issue and confirm whether or not the assignment of the Saudi Arabian male to A. thompsoni is correct.
As already pointed out by Anker (2022), several other types of colour pattern with parallel-running lines seem to correspond to further species, some of them closely related to A. thompsoni . For instance, a very distinctive colour pattern, hereafter type C pattern, exists in some snapping shrimps from Indonesia ( Fig. 9B, C View FIGURE 9 ; see also Debelius 2001: 150, upper photograph under “ A. ochrostriatus ”). The type C pattern differs from both pattern types of A. thompsoni (types A and B, as defined above), in the colour of the second to fifth pereiopods, which are deep blue with bright yellow markings near articulations (not yellow, as in A. thompsoni ), and in the background colour and general pattern of the major and minor chelae, which are dark greyish-brown or brown-green with pale bluish blotches and spots (not yellowish with longitudinal red lines / blotches, as in A. thompsoni ). The shrimps of the type C pattern possibly correspond to Alpheus sp. from the northern Red Sea in Karplus et al. (1981: fig. 2H, black-andwhite photograph in low resolution) and may well represent a further undescribed taxon, although several other species of the A. brevirostris group need be examined and compared. In the same publication of Debelius (2001: 150, also under A. ochrostriatus ), the middle photograph shows a pink-reddish shrimp with red longitudinal lines, which is generally similar to the type B pattern of A. thompsoni . This shrimp has a dull pink (instead of yellowish) background, pink-reddish legs (not yellow as in types A and B, or blue with yellow spots as in type C) and pinkish antennal flagella ( Fig. 9D View FIGURE 9 ; see also Anker 2022: fig. 8E). This colour pattern, hereafter the type D pattern, probably represents either A. mannarensis or a closely related undescribed taxon with affinities to both A. mannarensis and A. thompsoni ( Anker 2022; but see Miya’s view of colour pattern variation in Banner & Banner 1981). Further complicating the issue is the presence in the Red Sea of a dark red Alpheus sp. with a balaeniceps minor chela ( Fig. 9E, F View FIGURE 9 ), and with a colour pattern similar to that of A. mannarensis and the above-defined type D pattern. This colour pattern, hereafter the type E pattern, is defined by numerous, closely parallel-running lines of dark-red colour, the purplelilac antennal flagella and the uniform dark red-purple chelae ( Fig. 9F View FIGURE 9 ; see also Minemizu 2013: 103, Alpheus sp. 6 ). Although the species with the type E pattern was not included in Karplus et al. (1981), Dr. I. Karplus was aware of its presence and had some colour slides of it (I. Karplus, pers. comm.). The type E pattern is also reminiscent of the colour pattern of A. fenneri , although the latter species’ body is uniform orange brown, apparently without red lines ( Bruce 1994: fig. 5). In summary, the identification of goby-associated Alpheus spp. with the colour pattern types C–E will only be possible after the collection of photo-vouchered material the Red Sea and elsewhere in the Indo-West Pacific.
| FLMNH |
Florida Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Alpheus thompsoni Anker, 2022
| Anker, Arthur 2022 |
Alpheus sp. 4
| Minemizu, R. 2013: 102 |
Alpheus sp.
| Kuiter, R. H. & Debelius, H. 2009: 151 |
Alpheus “ ochrostriatus ”.
| Jaafar, Z. & Randall, J. E. 2009: 29 |
| Anker, A. 2000: 3 |
Alpheus djiboutensis
| Yu, H. P. & Jeng, M. S. & Chan, T. Y. & Ho, P. H. & Shy, J. Y. 1996: 35 |
Alpheus ochrostriatus
| Karplus, I. & Thompson, A. R. 2011: 591 |
| Poupin, J. 2010: 33 |
| Randall, J. E. 2005: 513 |
| Randall, J. E. & Shao, K. T. & Chen, J. P. 2003: 512 |
| Debelius, H. 2001: 150 |
| Debelius, H. 1997: 203 |
| Karplus, I. & Szlep, R. & Tsurnamal, M. 1981: 6 |