Thyasira methanophila, Oliver & Sellanes, 2005
|
publication ID |
https://doi.org/10.11646/zootaxa.1092.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:11F595CF-C728-4530-8362-32D585A0C8AC |
|
persistent identifier |
https://treatment.plazi.org/id/039B8788-A17B-FFF8-FEB7-FDF54369FA45 |
|
treatment provided by |
Felipe |
|
scientific name |
Thyasira methanophila |
| status |
sp. nov. |
Thyasira methanophila new species
Material Examined
Holotype. A live collected shell, Station AGT 7, 40 nautical miles NW off the Bay of Concepción, southcentral Chile; 36°22.15 S, 73°42.85 W; 780m depth. MNHNCL 201646. GoogleMaps
Paratypes. A live collected shell, Station AGT 13, 40 nautical miles NW off the Bay of Concepción, southcentral Chile; 36°22.15 S, 73°42.85 W; 780m depth NMW.Z. 2005.4.2 GoogleMaps . 3 valves + fragments, June 2004, 40 nautical miles NW off the Bay of Concepción , southcentral Chile; 36°22.15 S, 73°42.85 W; 780m depth MNHNCL 201647. Station AGT 6, 9 valves + fragments, 40 nautical miles northwest off the Bay of Concepción, southcentral Chile; 36°22.15 S, 73°42.85 W; 780m depth MNHNCL 201648 and NMW.Z. 2005.4.3 GoogleMaps .
Measurements
Holotype; length 20.5 mm, height 18.9 mm, tumidity (both valves) 11.9 mm. Summary statistics of morphometric ratios are presented in Table 1.
Description
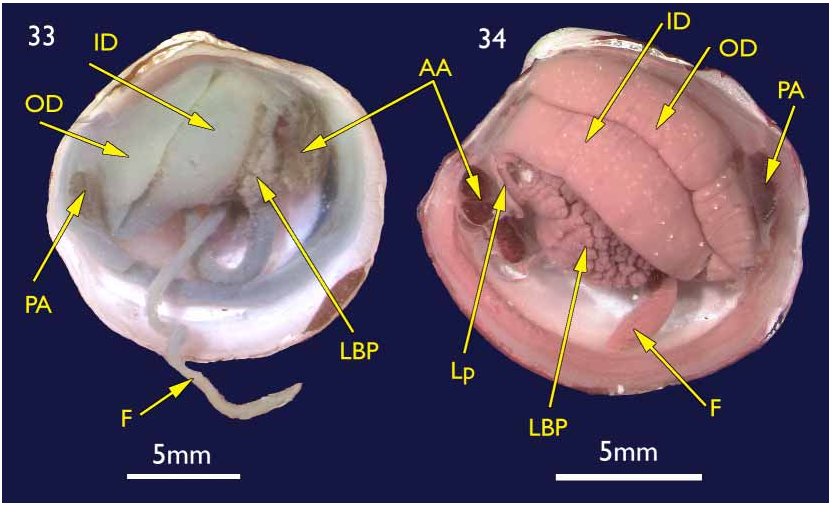
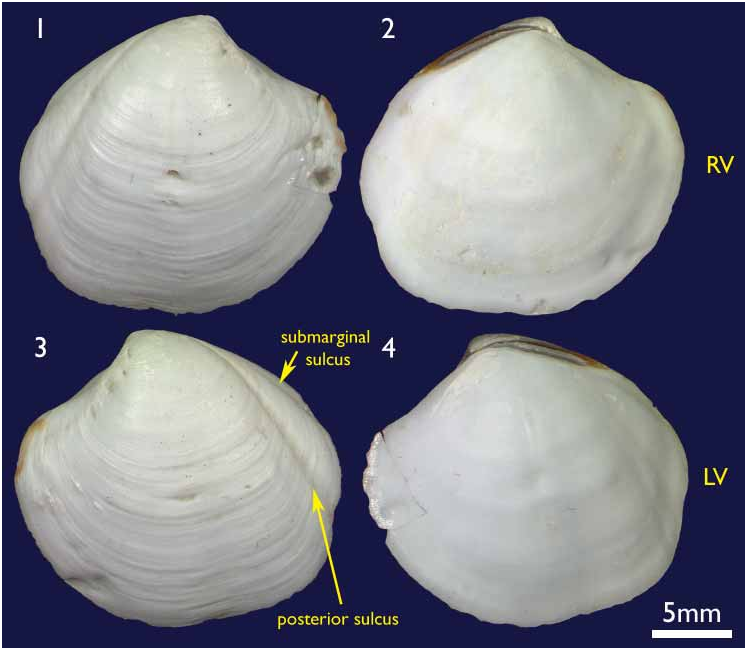
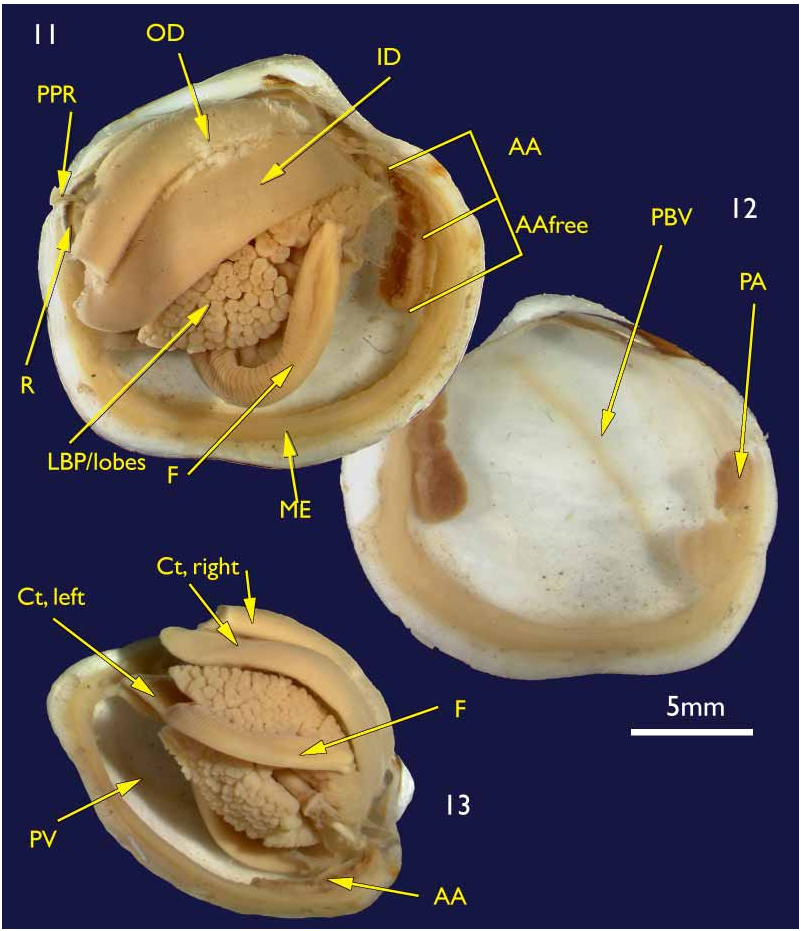
The shell. ( holotype Figures 1–4 View FIGURES 1–4 ; paratypes 5–10) To 30 mm in length. Thin. Equivalve. Relatively tumid (Length to Tumidity of both valves ratio, Mean = 1.6). Outline inequilateral to subequilateral; beaks distinctly towards the anterior or just towards the anterior; ovate slightly longer than high to height equal to length (Length to Height ratio, Mean = 1.1); umbos prominent, beaks slightly prosogyre; lunule relatively large (Length:Lunule length, Mean = 3.8), sunken and demarcated by a low rounded ridge; junction of lunule margin and anterior ventral margin subacute to narrowly rounded, distinguished in some as the termination of a very weak anterior ridge marking the aperture of the pedal siphon; anterior – ventral margin distinct in some as a rather straight, steeply sloping edge merging with the rounded ventral margin but in others the anterior ventral and ventral margins forming a continuous rounded curve; posterior margin with a distinct but shallow posterior sinus; marginal sinus very small, mostly obsolete; posterior dorsal (ligament) margin long, sloping steeply from umbo. Posterior sulcus distinct but narrow and shallow; submarginal sulcus ( Fig. 3 View FIGURES 1–4 ) long, very narrow (Height to Submarginal sulcus length ratio, Mean = 1.5), auricle absent; median area of valve slightly flattened to distinctly flattened. Ligament set on a deep resilium, deeply sunken although still visible externally. Hinge lacking teeth. Sculpture of irregularly spaced, comarginal lines and growth stops with irregular damage marks; submarginal sulcus with irregular transverse ridging ( Fig. 8 View FIGURES 5–10 ). Periostracum thin, straw coloured, finely wrinkled. Anterior adductor scar long, free portion half the length (Scar length to free part length ratio, Mean = 2.1) and just diverging from pallial line or continuous with pallial line ( Fig. 9 View FIGURES 5–10 ). A scar running from below the lunule margin to the posterior ventral margin (pallial blood vessel scar) is visible in some. Anatomy: Mantle thin, transparent with the pallial blood vessel showing as a dark line running diagonally across from the anterior dorsal to posterior ventral ( Fig. 12 View FIGURES 11–13 ). Mantle edge thick, no obvious proliferation of glandular tissue on its anterior inner edge ( Fig. 11 View FIGURES 11–13 ). Anterior adductor scar long, free portion diverging from the mantle edge ( Fig. 11 View FIGURES 11–13 ). Mantle fusion absent except at the posterior ventral margin where the gill terminates. Dorsal to this is a short smooth opening, the exhalent aperture. Posterior adductor muscle oval, about one third the size of the anterior adductor muscle.
Foot greatly elongate, vermiform, with a muscular ring at the junction with the visceral mass; heel indistinct ( Fig. 15 View FIGURES 14–15 ). Posterior pedal retractors small but considerably larger than the anterior pedal retractors.
Ctenidia ( Figs 11 & 13 View FIGURES 11–13 ) composed of both inner and outer demibranchs, both as thick fleshy lamellae, outer demibranch is less than half the depth of the inner.
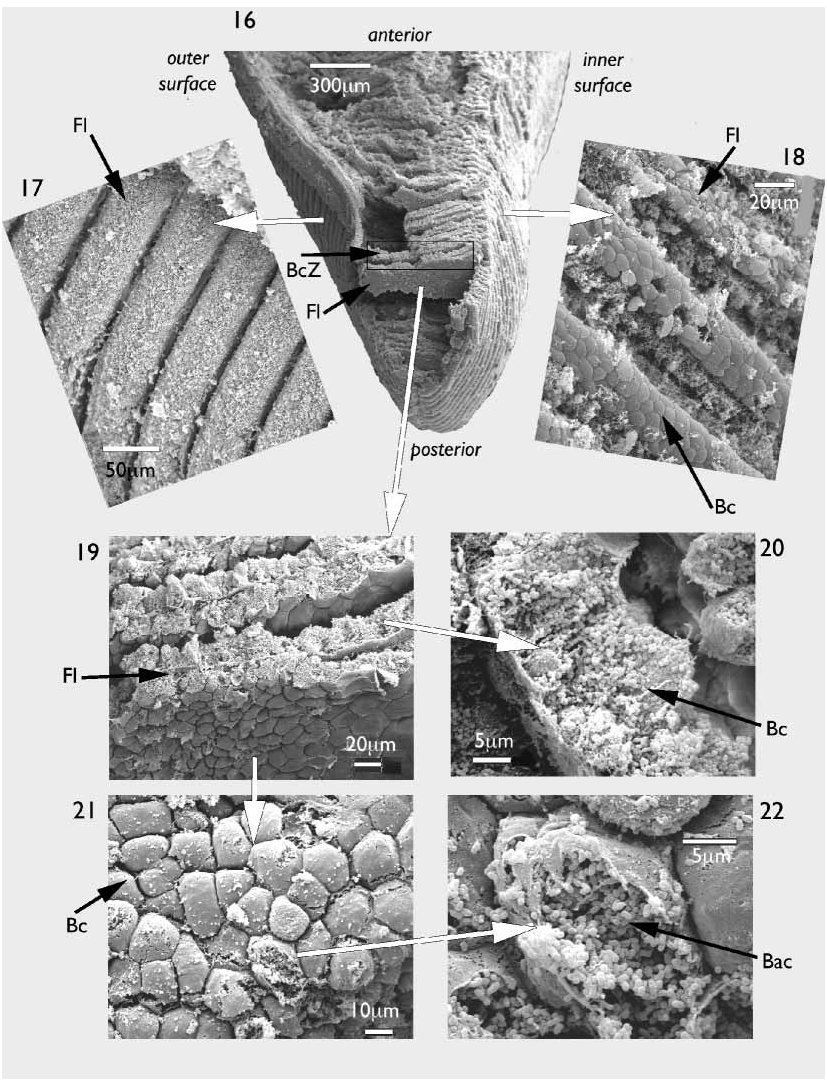
A scanning electron microscopy examination of the ctenidia revealed both the microstructure and distribution of the symbiotic bacteria. Demibranchs thick ( Fig. 16 View FIGURES 16–22 ), bacteriocyte zone relatively very long ( Fig. 16 View FIGURES 16–22 ). Filaments laminar in form ( Figs 16, 19 View FIGURES 16–22 ), ascending and descending arms are distinguishable only at the dorsal margin of the demibranch. Frontal surfaces of each filament heavily ciliated on the outer faces ( Fig. 17 View FIGURES 16–22 ), but less so on the inner faces ( Fig. 18 View FIGURES 16–22 ). Anterior and posterior faces of each laminar filament have a packed polygonal appearance ( Figs 19, 21 View FIGURES 16–22 ), these polygons also on the abfrontal faces of the filaments ( Figs 17, 18 View FIGURES 16–22 ) but obscured by cilia. Polygons are the bacteriocytes as can be seen in transverse section ( Fig. 20 View FIGURES 16–22 ) or where they have ruptured ( Fig.22 View FIGURES 16–22 ). Each bacteriocyte densely packed with short rod shaped bacteria, each approximately 0.5 m in length ( Fig.22 View FIGURES 16–22 ).
Labial palps partly damaged, but sorting ridges not apparent.
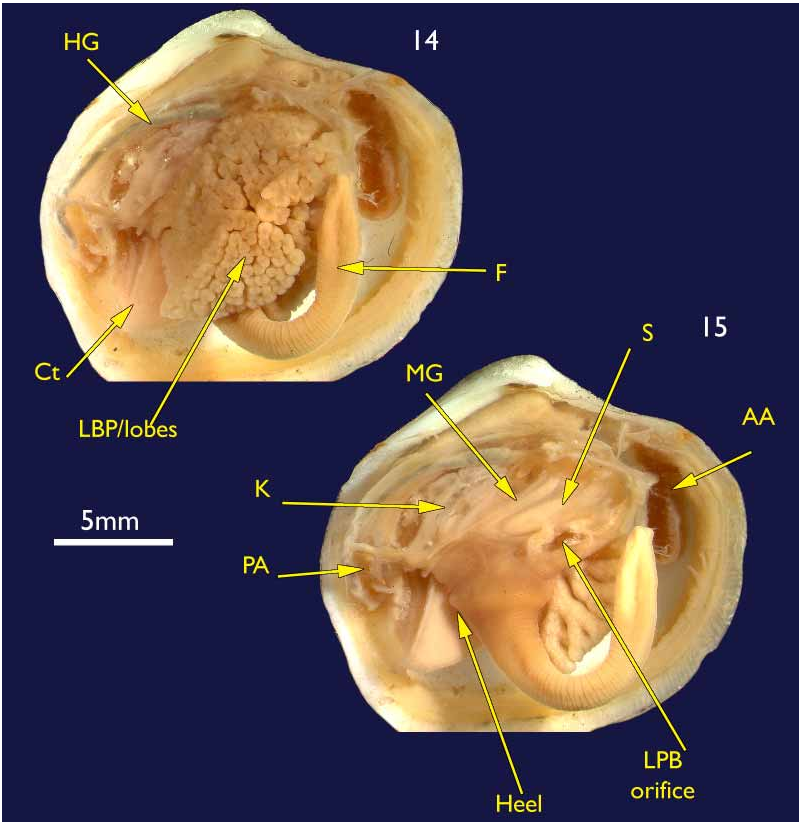
Lateral pouches very large, extensively lobed ( Fig. 14 View FIGURES 14–15 ). Lobes flat ended, roughly cuboidal in shape. Lobes arise from large, single orifice at base of stomach ( Fig. 15 View FIGURES 14–15 ).
Mouth could not be seen due to damage; oesophagus short; stomach cylindrical in a transverse longitudinal orientation ( Fig. 15 View FIGURES 14–15 ); mid gut a simple but tight loop and lies over the stomach before looping posteriorly into the straight hind gut and rectum ( Fig. 15 View FIGURES 14–15 ).
Kidneys large, situated below hind gut ( Fig. 15 View FIGURES 14–15 ). A large, white, powdery mass was present in the lumen.
Derivation of name. methanophila from methane and Latin suffix phila, meaning to like. Refers to the methane seep habitat.
Biotope. Sticky dark grey mud smelling of sulphide. Carbonate crusts are also typical features of the area and gas hydrates were retrieved from subsurface sediments at a nearby site.
Remarks
Generic placement. The generic definitions within the Thyasiridae have been recognised to be problematic ( Payne & Allen 1991; Oliver & Killeen 2002). In this instance, the anatomical characters of: both demibranchs present, extensive lobing on lateral pouches, elongate foot with indistinct heel and the shell characters of lack of hinge teeth and presence of a posterior sulcus all indicate the genus Thyasira sensu lato, as defined by Payne & Allen (1991).
The subgenus Parathyasira was used by Payne & Allen (1991) and Oliver & Killeen (2002), and is defined by the shell characters of a cleft escutcheon with no auricle (no submarginal sulcus) and a flattened rather than sulcate posterior margin. These latter characters are not shared by T. methanophila .
One genus not considered by Payne & Allen (1991) or Oliver & Killeen (2002) was Maorithyas Fleming, 1950 . This genus was erected for a relatively large New Zealand species exhibiting weak development of the posterior sulci, a condition that could be applied to the current species and other southern ocean species, such as T. falklandicum Smith, 1885 . We have examined the type species of Maorithyas , M. marama Fleming, 1950 , and give the following observations.
The shell ( Figs 23–25 View FIGURES 23–26 ) is rather inflated, roundly ovate with rounded margins, in some there is a short anterior dorsal margin indicating the exit of the pedal siphon and the posterior margin is weakly sinuate. The posterior sulcus is very shallow, and there is no median flattening and no auricle. There is a very narrow incised line (= submarginal sulcus) demarcating the escutcheon (Fig. 28). There are no hinge teeth, and the ligament is deeply inset but visible from the dorsal view.
The anatomy (Figs 30–32) is consistent with that of Thyasira ; the foot is vermiform with a very small heel. The ctenidia consist of both outer and inner demibranchs, the former less than half the depth of the latter. The lateral body pouches are large and multilobed, the ends of the lobes cuboidal in form. The mantle edge is thick and is free except at the termination of the ctenidia and dorsal to this point is a short, smooth apertured, exhalent siphon. The labial palps are small and without sorting ridges. The oesophagus is short, the stomach cylindrical and the midgut coiling simply over the stomach before bending posteriorly into the hind gut and rectum.
Thyasira falklandicum ( Figs 35–38 View FIGURES 35–38 ) has a similar shell form to M. marama in having a weak posterior sulcus and weak development of the escutcheon (Fig. 29) but is rather compressed. Anatomically ( Fig. 33 View FIGURE 33 ), this species shares the gross features of M. marama and again these are consistent with Thyasira , as exemplified by T. sarsi (Fig. 34).
FIGURE 34. Thyasira sarsi , North Sea, NMW.Z.1996.028, Gross anatomy after removal of left mantle.
From the anatomy there are no characters that warrant generic separation of the southern ocean species considered here. On shell characters, the genus Maorithyas is defined by the weak posterior sulcus and faint demarcation of the escutcheon. The expression of these characters varies from species to species and there is a continuum from weakest development in T. falklandicum (Fig. 29)› T. marama (Fig. 28)› T. methanophila (Fig. 27)› T. fuegiensis › T. sarsi . The use of shell characters to define genera in such a series becomes subjective and we cannot support Maorithyas , even at the subgeneric level.
Molecular data on thyasirids is scant but a preliminary study by John Taylor (pers. comm.) has shown a closer affinity of T. sarsi to T. methanophila than to T. flexuosa ( type species of the genus Thyasira ). This is a suggestion that T. sarsi does not perhaps belong to the same clade as T. flexuosa and may actually support a separate genus. However, until molecular data are available for the type species of nominal thyasirid genera we feel it unwise to place T. methanophila anywhere other than in Thyasira s.l.
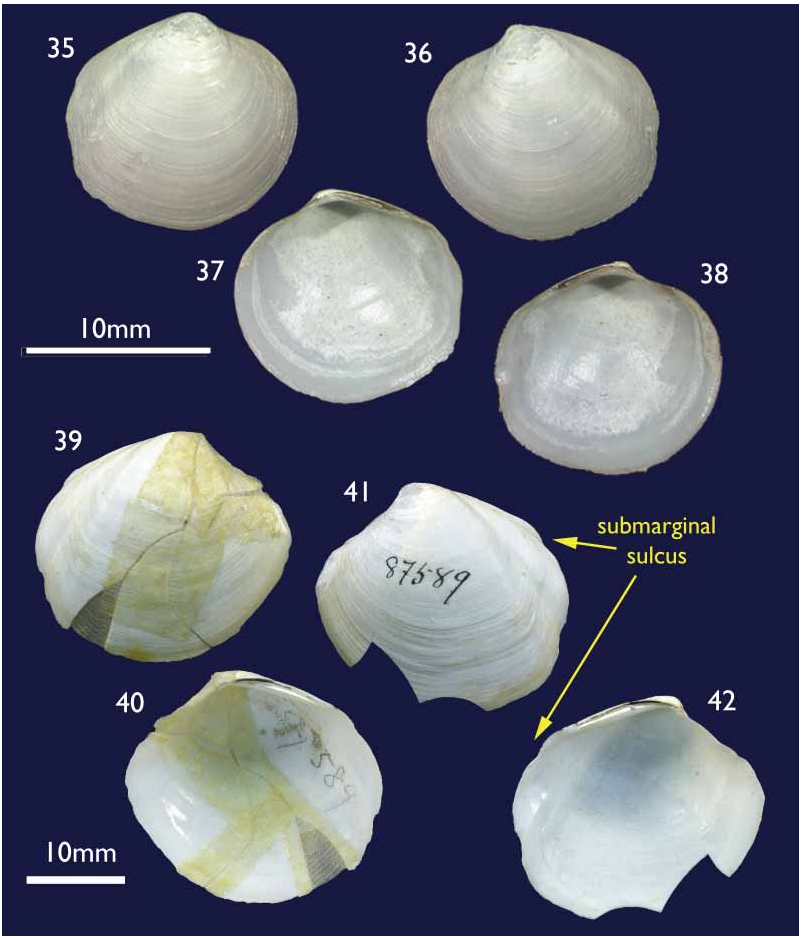
Species comparisons. Relatively few species of Thyasiridae have been described from the southern oceans or pacific coast of South America and relatively few species in general (excluding species of Conchocele ) reach the size of T methanophila . The most frequently encountered Thyasira on the Chilean coast is T. tomeana Dall, 1901 , but it is a small distinctly bicarinate species. Another small species is T. magellanica Dall, 1901 , and has virtually no posterior sulcus. It was described from a single valve and may represent juvenile T. falklandicum . Larger species from the Magellanic region are T. falklandicum ( Smith, 1885) and T. fuegiensis ( Dall, 1889) . Thyasira falklandicum ( Figs 35–38 View FIGURES 35–38 ) is conclusively distinguished by its lack of any demarcation of the escutcheon. Thyasira fuegiensis (Figs 39–42) by contrast is similar but it was described from reconstructed fragments of a single shell and remains known only from that shell collected in the Straits
FIGURES 39–42. Thyasira fuegiensis Dall, 1889 , Holotype, Magellan Straits, US Fish Com. Station 2779, 77.5 fathoms, USNM 87589. Figs 39–40 external and internal of right valve; Figs 41–42 external and internal of left valve.
of Magellan at a depth of 77.5 fathoms ( 170 m). This species differs in having a distinct auricle and submarginal sulcus giving a bicarinate outline to the posterior margin. The closest approximation to T. fuegiensis is the North Atlantic T. sarsi , which can reach similar dimensions. Supporting findings for the separation of T. methanophila and T. fuegiensis come from the high levels of endemism reported for seepinhabiting species ( Sibuet & Olu 1998). The evidence so far obtained proves that this statement is also true for the Concepción methane seepage area, since at least three more new species, a Calyptogena ( Sellanes & Krylova 2005) , an Archivesica (under study) and a Lucinoma ( Holmes et al. 2005) have been confirmed for the area. Further support comes from the faunistic separation of the magellanic and southcentral Chilean regions that occurs around 45° S and coincides with the diverging Cape Horn and Humboldt current systems ( Fernandez et al. 2000; Valdovinos et al. 2003).
Given the degree of endemism reported for seep and vent organisms ( Sibuet & Olu 1998) affinity with thyasirids from these ecosystems were also considered. The most frequent records of thyasirids from seeps are of Conchocele (see below) and the occurrences of Thyasira s.l. are few.
In the southeastern Pacific there are no records but in the northeastern Pacific a large thyasirid has been reported from the Cascadia Basin (E. Southward pers.comm.). This thyasirid is large, reaching 26 mm, and is polygonal in outline, resembling the genus Axinus to some degree and therefore quite unlike the Chilean shells.
In the Atlantic Ocean, T. sarsi has been reported from methane seeps in the North Sea ( Dando et al. 1994) but this species is also known from vegetationderived organic rich environments in Norwegian fjords ( Southward 1986). This species may reach 30 mm in length, has weakly developed posterior sulci but has a low auricle. Two other large Atlantic thyasirids are known but remain undescribed (Oliver unpubl. data). Southward et al. (2001) illustrate a species with weak posterior sulci and a rather reduced and roundly acute anterior from the Logatchev hydrothermal site on the MidAtlantic Ridge at 14°45’N and 3038 m depth. The illustrated shells are over 20 mm in length and are placed in the subgenus Parathyasira .
Another species was found inhabiting the bean and seed cargo of the wreck of the Francois Vieljeux off Vigo, Portugal, at 1160 m depth. This species resembles the above in outline and the escutcheon is excavated but very narrow and without an auricle.
Thyasira oleophila Clarke, 1989 , was described from the Louisiana oil and gas seeps from depths around 500 m. This is a large species reaching 22 mm in length and is almost devoid of posterior sulci. This species is not expanded anteriorly and is reminiscent of T. sarsi .
A number of Thyasira species are reported from Japanese waters but their association, with seeps or vents, is not always clear. Most recently Okutani et al. (1999) described two new species of Thyasiridae from hadal depths in assumed chemosynthetic assemblages. Maorithyas hadalis Okutani, Fujikura & Kojima, 1999 is large, to 36 mm in length, with weak posterior sinus and escutcheon. The foot is described as being “unmodified” and therefore quite unlike most thyasirids and doubtfully belonging to Maorithyas or Thyasira s.s. The second species, Parathyasira kaireiae Okutani, Fujikura & Kojima, 1999 is much smaller, to 12 mm in length, and is very similar in outline to the two undescribed Atlantic species and like them has been placed in Parathyasira . Methane seeps are also known from Sagami Bay ( Sibuet & Olu 1998) and a number of bathyal thyasirids have been recorded from this area. No direct associations have been made and the species have been assigned to a variety of genera including Thyasira ( T. imamurai Okutani, 1962 ), Axinulus ( A. kelliaeformis Okutani, 1962 , and A. obliqua Okutani, 1968 ) and Leptaxinus ( L. elegans Okutani, 1968 ). There are no anatomical data to confirm these generic placings and the only large species with weak posterior sulci is A. obliqua .
Affinities. Comparisons of T. methanophila with southern ocean taxa and those known from seep and vent communities suggest that the affinity lies with other species from the same region rather than with species associated with chemosynthetic communities. Thyasirids in general are wide ranging in the degree of their bacterial association and inhabit a wide range of ecosystems. Indeed Thyasira s.l. is not frequently found in chemosynthetic communities ( Sibuet & Olu 1998). The wide range of shell morphologies noted in those species that do associate with seeps and vents suggests that they do not belong to a single clade. The only shell feature in common is the relatively large size, and this may be due to the trophic regime in which they live
Anatomy. The anatomy of T methanophila is consistent with other large species of Thyasira and there are no gross features showing any modification to the seep environment. The fine structure of the filaments, disposition of bacteriocytes and bacterial density all indicate a very heavy dependence on symbiosis. The long bacteriocyte zone is similar to that seen in T. sarsi ( Southward 1986) , a species with known affinities to extreme reducing environments ( Dando et al. 1994). The bacteria observed in T. methanophila all appear to be of one type, and this is also the situation in T. sarsi (Dando pers. comm.) Although T. methanophila could be described as a deepwater species, it differs from those described by Southward (1986) in having only one type of bacterium.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.