Victorgorgia macrocalyx ( Nutting, 1911 ) Moore & Alderslade & Miller, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4304.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:3D557C94-0783-4C39-80C3-9C321DA94800 |
|
DOI |
https://doi.org/10.5281/zenodo.6015389 |
|
persistent identifier |
https://treatment.plazi.org/id/E7B6CAB2-980B-40F5-A380-EDFB8C94FF22 |
|
taxon LSID |
lsid:zoobank.org:act:E7B6CAB2-980B-40F5-A380-EDFB8C94FF22 |
|
treatment provided by |
Plazi |
|
scientific name |
Victorgorgia macrocalyx ( Nutting, 1911 ) |
| status |
comb. nov. |
Victorgorgia macrocalyx ( Nutting, 1911) new combination
( Figs. 120–126 View FIGURE 120 View FIGURE 121 View FIGURE 122 View FIGURE 123 View FIGURE 124 View FIGURE 125 View FIGURE 126 )
Suberia macrocalyx Nutting, 1911: 15 View in CoL , Pl. III Fig. 3, 3 View FIGURE 3 a, Pl. XI Fig. 5 View FIGURE 5 a–c.
Semperina macrocalyx ( Nutting, 1911) : Kükenthal 1916: 174; 1919: 51, 57; 1924: 22; Thomson & Dean 1931: 192, Pl. XIV Fig. 3 View FIGURE 3 , Pl. XXIV Fig. 6 View FIGURE 6 ; Stiasny 1937: 35, 119, Pl. IV Fig. 34 View FIGURE 34 , Textfigure J.
Anthothela macrocalyx ( Nutting, 1911) : Verseveldt 1942: 170, Fig. 5 View FIGURE 5 .
Iciligorgia macrocalyx ( Nutting, 1911) : van Ofwegen et al. 2000–2007 http://www.marinespecies.org/ aphia.php?p=taxdetails&id= 290176 accessed May 2017.
Material examined. Holotype: ZMA COEL 3280 , near Manado , Celebes, Indonesia, Siboga Expedition , stn. 122, 1.975°N, 125.158°E, depth 1264– 1165 m, 17th July 1899. GoogleMaps
Description:
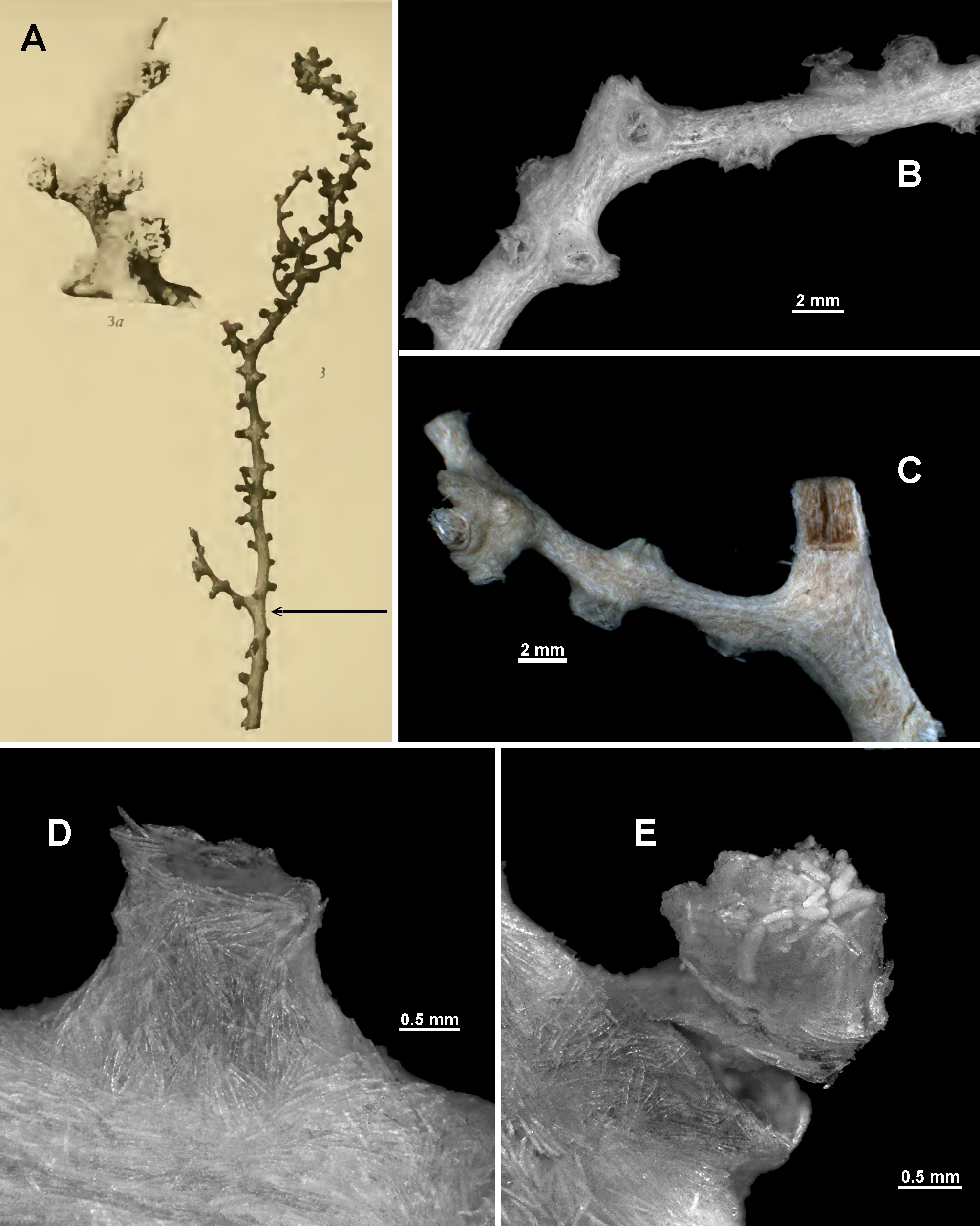
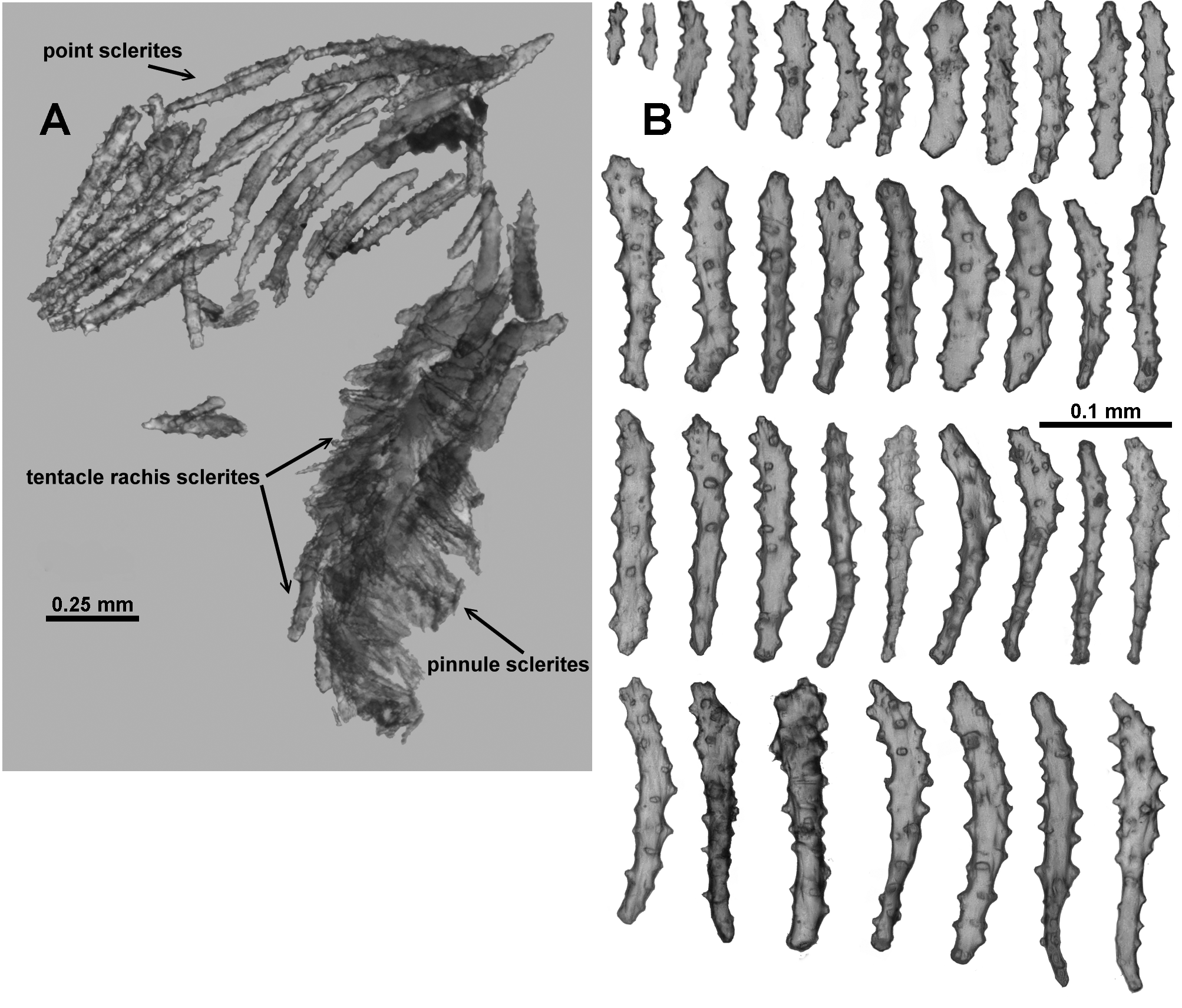
Colony form: In his description, Nutting describes the holotype as an incomplete specimen consisting of an “erect stem with short scattered branches” that is 135 mm in length ( Nutting 1911) (reproduced here Fig. 120 View FIGURE 120 A). Two fragments of the holotype were examined for this study, one of which can be confidently located on the figure of the holotype by its substantial bifurcation ( Fig. 120 View FIGURE 120 C). It can therefore be deduced that the exposed medulla is part of a substantial branch. The other fragment is a piece of branch or stem which cannot be reliably positioned on the figure of the holotype ( Fig. 120 View FIGURE 120 B). Unfortunately, these two fragments are in a very poor condition with a single remaining partially attached polyp between them plus two detached polyp heads. Nevertheless, by combining an examination of these fragments with Nutting’s original description and the subsequent descriptions and figures of the holotype by Stiasny (1937) and Verseveldt (1942) it is possible to provide a reasonably complete re-description.
The original colony clearly had at least one main branch with a few short side branches, although they appear to be broken in places and the holdfast is missing ( Fig. 120 View FIGURE 120 A). Nutting states that the main “stem” diameter is 3 mm and this is confirmed here. It appears from Nutting’s figure that there are some anastomoses present in the colony, with possibly two loops identifiable. However, considering Nutting and Stiasny do not mention any anastomoses, and Verseveldt specifically says “there are no anastomoses” the colony loops are considered an artefact of the figure. According to Nutting there were six branches emanating from all sides of the main branch and the calyces were present on all available parts of the colony. Nutting states that “the calyces are irregularly distributed on three sides of the proximal parts of the stem and branches and on all sides of the distal parts of the colony”. However, Stiasny and Verseveldt both refute this, finding calyces and polyps on all sides throughout the colony. The fragments examined here have remnants of calyces spread evenly along and on all sides of the branches with up to 5 mm between them, although they are often closer than that ( Fig. 120 View FIGURE 120 B, C).
In its original condition, the holotype had intact branch tips where, according to Nutting, the calyces “form definite clumps or clusters with the individual calyces averaging about 1.5 mm apart”. There is a small clump present on one of the fragments examined here, with two or three calyces crowded together ( Fig. 120 View FIGURE 120 C).
Colour: Nutting mentions the colour as “very light yellowish brown”. The holotype fragments are now cream in alcohol.
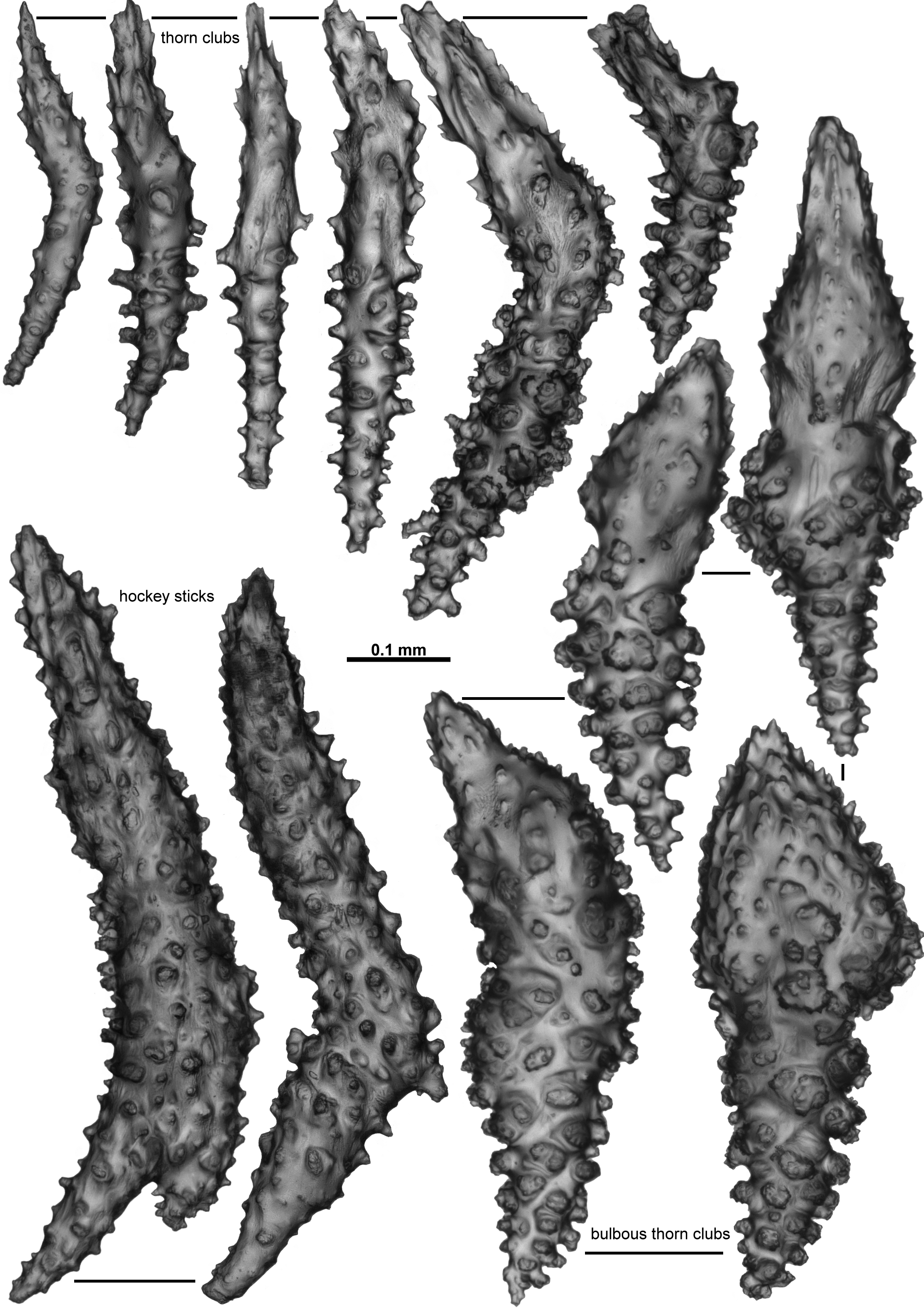
Calyces and polyps: There are many calyces remaining on the holotype fragments but they are fragile and easily damaged with many of them already heavily impacted. Those in better condition are conical to cylindrical, usually 1–2 mm high and 1.5–2 mm wide, with a thin layer of sclerites arranged longitudinally to obliquely on their side walls ( Fig. 120 View FIGURE 120 D). There is some tendency towards an en chevron arrangement of the calyx sclerites and Nutting states this is more pronounced around the calyx lip where the sclerites form “eight angular points around the margin”. Some hint of an en chevron arrangement was also visible in the sclerites at the base of the calyx figured here ( Fig. 120 View FIGURE 120 D).
The single remaining polyp on the fragments is partially retracted such that the head rests on the calyx lip ( Fig. 120 View FIGURE 120 E). It projects approximately 1.2 mm above the calyx and is 1.8 mm wide. Nutting states that most of the polyps are partly retracted like this and specifically that the “polyps are retractile”. The polyp head is covered in sclerites arranged in a collaret and points and has sclerites which are particularly large and dense arranged along the tentacle rachis ( Fig. 120 View FIGURE 120 E).
Medulla and Cortex: The medulla is made up of tightly packed, longitudinally arranged sclerites and is surrounded by an easily detached cortex approximately 0.2 mm thick ( Fig. 121 View FIGURE 121 A). The medulla and cortex are separated by parallel, longitudinal canals which join and anastomose so as to form a boundary space with attachments between the cortex and medulla only occurring occasionally. In Verseveldt’s paper (1942) he described the boundary canals in cross-section as “usually much flattened, on the cortex-side they are flat, on the medullaside they are rounder. Their height in a radial direction amounts to 0.05–0.11 mm, sometimes to 0.16 mm; the breadth is 0.18–0.20 mm ”.
Additionally, there is a cluster of 3 large, conspicuous coelenteric canals penetrating the centre of the medulla in the two fragments examined, with 2 or 3 other smaller and less distinct canals on the edge of the centre cluster ( Fig. 121 View FIGURE 121 A). The larger canals range from 0.2–0.4 mm in diameter and do not appear to significantly differ in diameter throughout the two fragments examined.
For those polyps positioned along the branches, the polyp cavities terminate abruptly at the medulla with an almost flat base visible at the base of the empty calyces. Due to the scarcity of remaining material, the arrangement of the canals at the tip of the branches was not investigated. Verseveldt bemoaned his inability to thoroughly investigate the canal system of the holotype, particularly that near the terminal polyps, and finishes with “In my opinion it will depend on the behaviour of the medullary canals with regard to the terminal zooids, whether for macrocalyx quite a new genus must be assumed.”
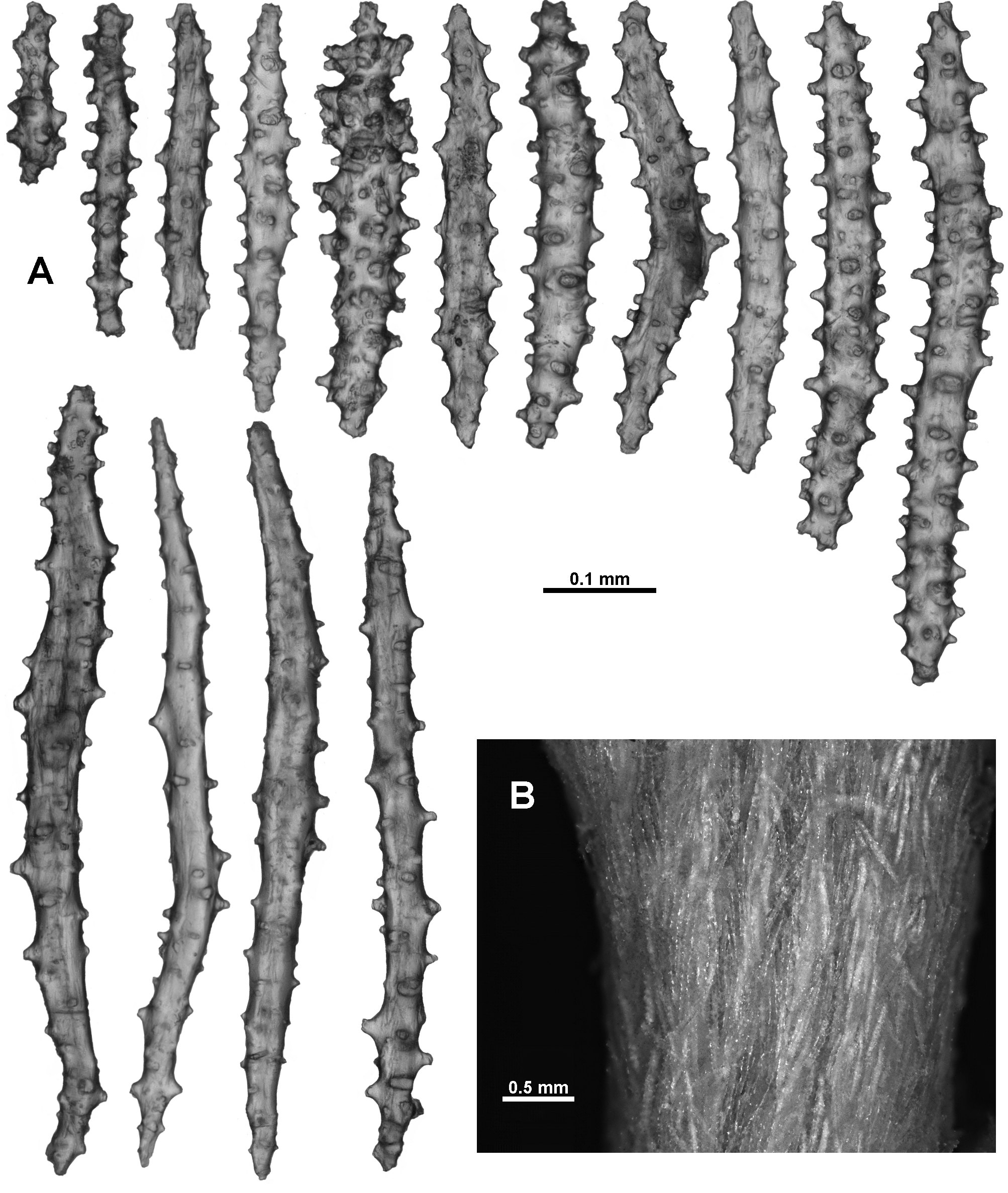
Sclerites: Unfortunately the remaining polyp does not provide a good example of the arrangement of the sclerites on the polyp head with many sclerites dislodged and damaged. However, both Thomson & Dean (1931) and Verseveldt (1942) describe a ring of approximately 6–10 sclerites arranged transversely forming a collaret and similar sclerites arranged en chevron to longitudinally forming eight distinct points. These sclerites are mostly simple sticks and spindles with a relatively sparse covering of tubercles, and range from 0.2–0.62 mm long ( Fig. 121 View FIGURE 121 B). Mixed with these in the distal region of the points (and crossing over into the tentacle rachis) are large, clubbed, warty sclerites ( Fig. 121 View FIGURE 121 C) arranged with their blunt clubbed ends towards the top of the points. Verseveldt specifically mentions that some sclerites from the points are “strong and club-shaped. I have not succeeded in finding the curious, thick and club-shaped spicules drawn by Thomson & Dean (1931, pl. XXIV fig. 6) and by Stiasny (1937, Text fig. Ja, b) anywhere either in cortex or medulla, they only occur in the anthocodiae.” These club-shaped sclerites have sparse, distinctly projecting, tall, conical tubercles. Very few of these clubbed sclerites were sampled here but those examined were approximately 0.35–0.65 mm long. Verseveldt stated that “most of them are 0.50–0.65 mm long, but shorter ones also occur (0.30 mm); the club-shaped end is 0.085–0.120 mm thick, without processes.”
Sclerites on the tentacle rachis are arranged longitudinally and are mostly short, fat rods with few, low tubercles ( Fig. 122 View FIGURE 122 A, B). The larger, bulkier rods are white and opaque, and clearly visible on the tentacle rachis. Some of the rods are slightly clubbed with a clump of tubercles at one end of the sclerites, and they basically lie longitudinally with the tuberculate head placed towards the end of the tentacles. Sclerite length grades continuously from 0.17–0.40 mm with most of the bulky rods between 0.23–0.40 mm. The shorter sclerites are usually placed closer to the tip of the tentacles ( Fig. 123 View FIGURE 123 A).
Many short, simple sticks and spindles and flat rods are crowded in the pinnules, all arranged longitudinally, and ranging from 0.04–0.23 mm long ( Fig. 123 View FIGURE 123 A, B). There are only very sparse tubercles on these sclerites with a tendency for some of the sclerites to have very slightly clubbed or expanded tips, with the tips arranged distally in the pinnules. There were no true josephinae clubs detected in the small sample available for examination. It may be that they are rare and were not sampled or it may be there are no josephinae clubs in the tentacles of this species.
No sclerites were detected in the pharynx.
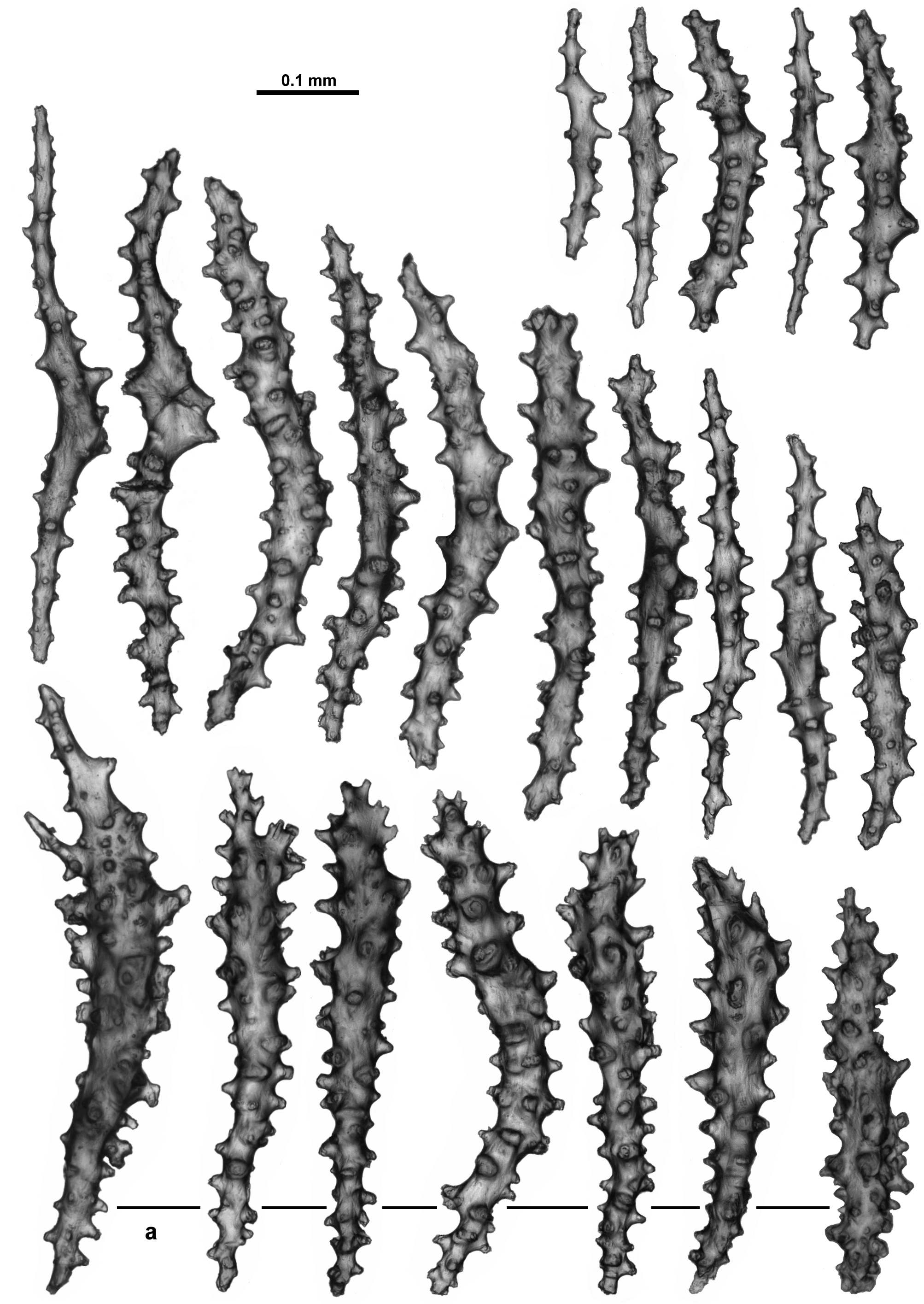
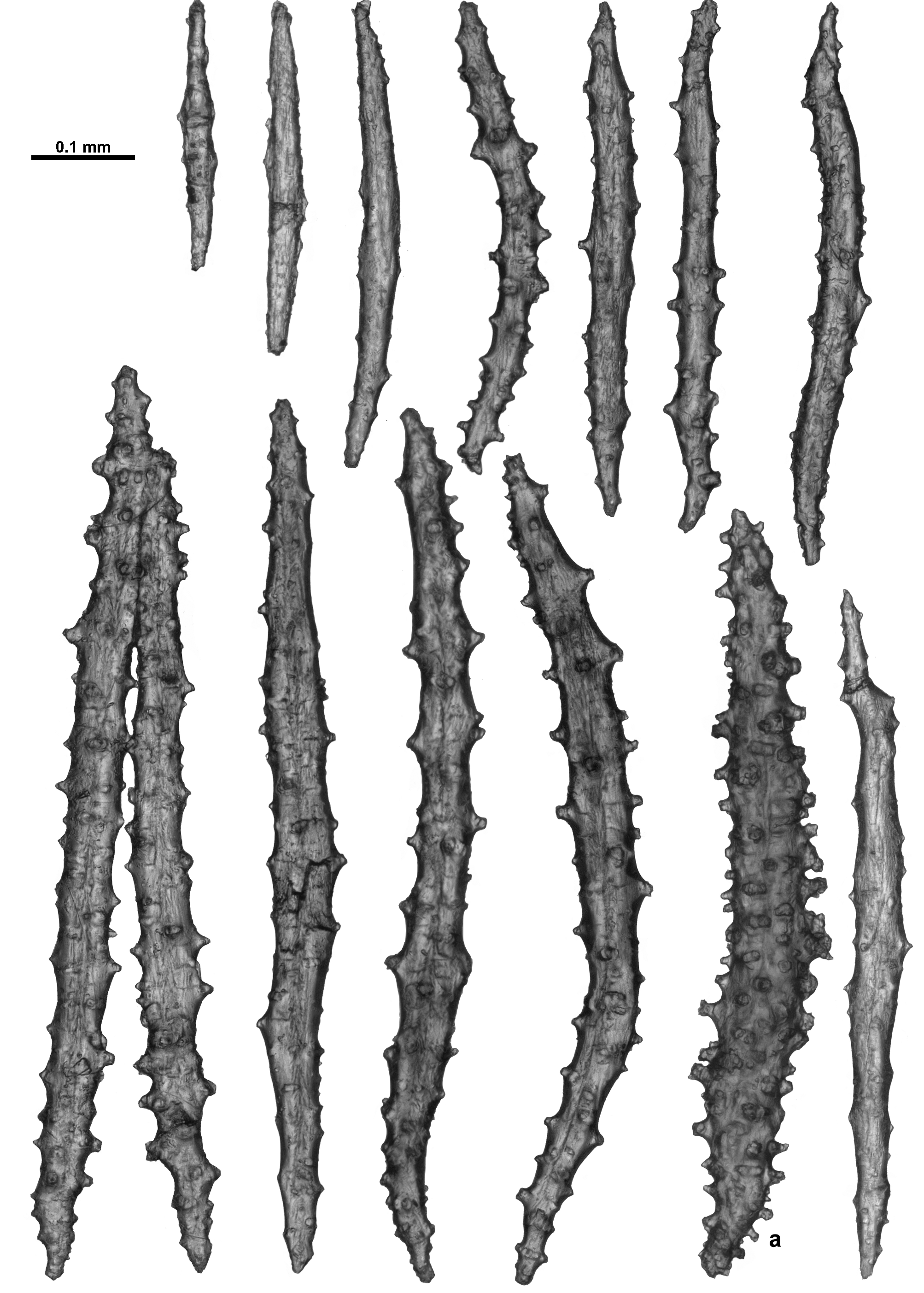
The calyx has only a single layer of sclerites arranged in indistinct en chevron arrangements up the wall. These sclerites are all straight sticks and spindles, some slightly thicker, with simple tubercles and short spines arranged haphazardly on the sclerites with no tendency for distinct asymmetry or clumping ( Fig. 124 View FIGURE 124 ). Length varies from 0.35–0.67 mm. Sclerites from the cortex are very similar ( Fig. 125 View FIGURE 125 A). They are arranged longitudinally along the branch in a thin layer and are usually 0.35–0.67 mm long although some smaller sclerites are present. There are faint longitudinal corrugations in the cortex, presumably mapping the boundary canals below ( Fig. 125 View FIGURE 125 B).
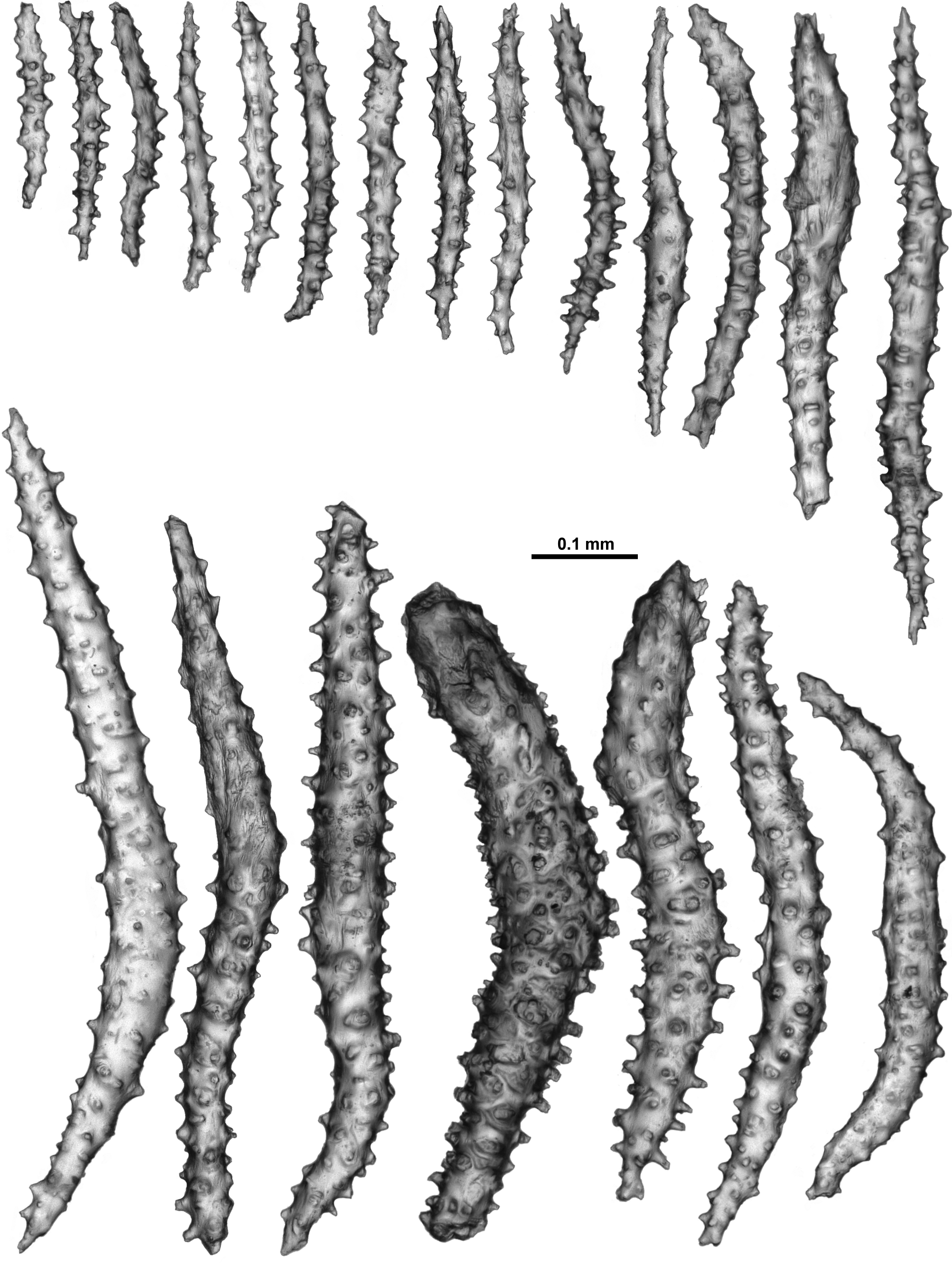
When magnified in transmitted light, most of the sclerites from the medulla are brown with a fibrous appearance. Similar to the calyx and surface, they are mostly straight sticks and spindles but many of them are smoother with very few tubercles ( Fig. 126 View FIGURE 126 ). There are also some with numerous tubercles ( Fig. 126 View FIGURE 126 a) but these are not as common, and there are some fused and branched sclerites as well. Length ranges from 0.27–0.90 mm, although, as is often the case with medulla sclerites, the longer sclerites may be underrepresented due to breakage.
Sclerites are all transparent under transmitted light except the bulky sclerites in the tentacles and points and most medulla sclerites, which tend to be brown.
Distribution: Indonesian archipelago
Depth: 1264–1165 metres.
Remarks: The state of the holotype is such that any decisions on the status of this species must be made with some caution. It is clear this species should not stay in the genus Anthothela due to the colony growth form, presence of large coelenteric canals in the medulla and the lack of sclerites in the pharynx. All of these characteristics plus the general form and arrangement of the sclerites indicate a placement in Victorgorgia . The main caveat however, is the apparent absence of josephinae clubs in the tentacles. These particular sclerites are common in other Victorgorgia species so the lack of them in V. macrocalyx n. comb. introduces a level of uncertainty to the reassignment. Given the limited material available for examination it is possible that the josephinae clubs are present but in small numbers and were simply missed during this necessarily limited analysis. The species is placed in Victorgorgia until new material can be examined.
This specimen is from deep waters off the coast of Indonesia. There has been very little sampling of this habitat and very little chance to collect more of this species. Additionally, the degree of connectivity of this area with other deep-sea areas is largely unknown thus the likelihood of recording this species in other places is, at this stage, unpredictable.
The presence of bulky, short rods on the tentacle rachis of V. macrocalyx n. comb. is the main feature distinguishing this species from most of the others in Victorgorgia . The most similar species is V. alba n. comb. which has few josephinae clubs, similar thick rods on the tentacles and bulky clubs in the points. The differences between the two species are specified in the Remarks section in the description of V. alba n. comb. (page 140)
V. josephinae and V. argentea n. comb. have many josephinae clubs in the tentacles, V. eminens n. sp. lacks any large, bulky sclerites in the points or the tentacles and V. nyahae n. sp. has sharply tipped thorn clubs in the points and tentacles.
| ZMA |
Universiteit van Amsterdam, Zoologisch Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Octocorallia |
|
Order |
|
|
SubOrder |
Scleraxonia |
|
Family |
|
|
Genus |
Victorgorgia macrocalyx ( Nutting, 1911 )
| Moore, Kirrily M., Alderslade, Philip & Miller, Karen J. 2017 |
Anthothela macrocalyx ( Nutting, 1911 )
| Verseveldt 1942: 170 |
Semperina macrocalyx ( Nutting, 1911 )
| Stiasny 1937: 35 |
| Thomson 1931: 192 |
| Kukenthal 1916: 174 |
Suberia macrocalyx
| Nutting 1911: 15 |