Titanonarke molini ( Jaekel, 1894 )
|
publication ID |
https://doi.org/ 10.1080/14772019.2017.1371257 |
|
publication LSID |
lsid:zoobank.org:pub:EDD6E170-CA64-4FFB-8DD1-AED2D61D5504 |
|
DOI |
https://doi.org/10.5281/zenodo.10911948 |
|
persistent identifier |
https://treatment.plazi.org/id/03E88796-2270-B04E-2565-EF19FB01F907 |
|
treatment provided by |
Felipe |
|
scientific name |
Titanonarke molini ( Jaekel, 1894 ) |
| status |
|
† Titanonarke molini ( Jaekel, 1894)
( Figs 2–16 View Figure 2 View Figure 3 View Figure 4 View Figure 5 View Figure 6 View Figure 7 View Figure 8 View Figure 9 View Figure 10 View Figure 11 View Figure 12 View Figure 13 View Figure 14 View Figure 15 View Figure 16 )
1860 † Narcine gigantea Molin : 585 [pro parte].
1894 † Narcine molini Jaekel : 111, pl. 3 [original occurrence of name, photograph and outline reconstruction].
1904 † Narcine molini Jaekel, 1894 ; Eastman: 27.
1905 † Narcine molini Jaekel, 1894 ; Eastman: 351.
1979 † Narcine molini Jaekel, 1894 ; Stanghellini: 38 [misspelt ‘ moloni’].
1980 † Narcine molini Jaekel, 1894 ; Blot: 344.
1987 † Narcine molini Jaekel, 1894 ; Cappetta: 161, fig. 138L.
1988 † Narcine molini Jaekel, 1894 ; Cappetta: 29.
1991 † Narcine molini Jaekel, 1894 ; Frickhinger: 211.
1993 † Narcine molini Jaekel, 1894 ; Cappetta et al.: 604.
1999 †‘ Narcine ’ molini Jaekel, 1894 ; Carvalho: 283, figs 107–111.
2010 † Titanonarke molini ( Jaekel, 1894) ; Carvalho: 185, figs 2, 5A, 6, 7.
2012b † Titanonarke molini ( Jaekel, 1894) ; Aschliman et al.: 33.
2012 † Narcine molini Jaekel, 1894 ; Cappetta: 410, fig. 401L.
2014 † Titanonarke molini ( Jaekel, 1894) ; Carnevale et al.: 41.
2014 † Titanonarke molini ( Jaekel, 1894) ; Claeson: 4.
2016c † Titanonarke molini ( Jaekel, 1894) ; Marram̀a et al.: 232.
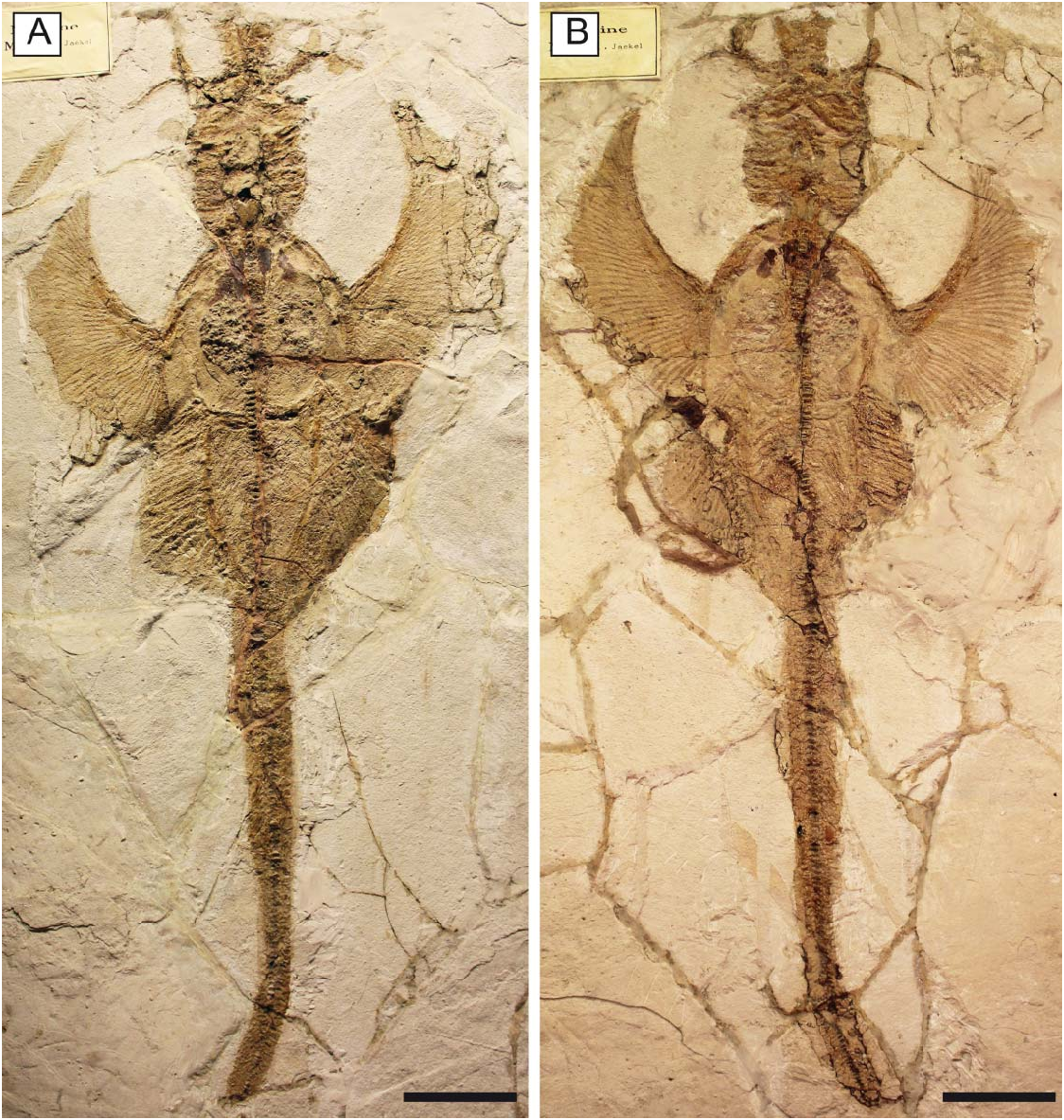
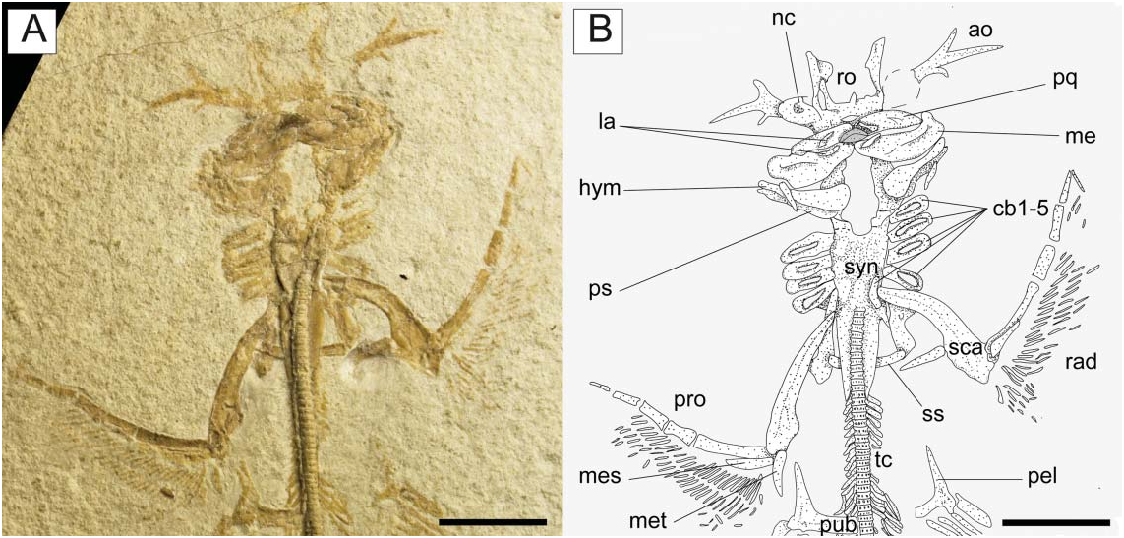
Holotype. MGP-PD 26275 /6, nearly complete articulated skeleton in part and counterpart ( Fig. 2 View Figure 2 ), 816.8 mm SL.
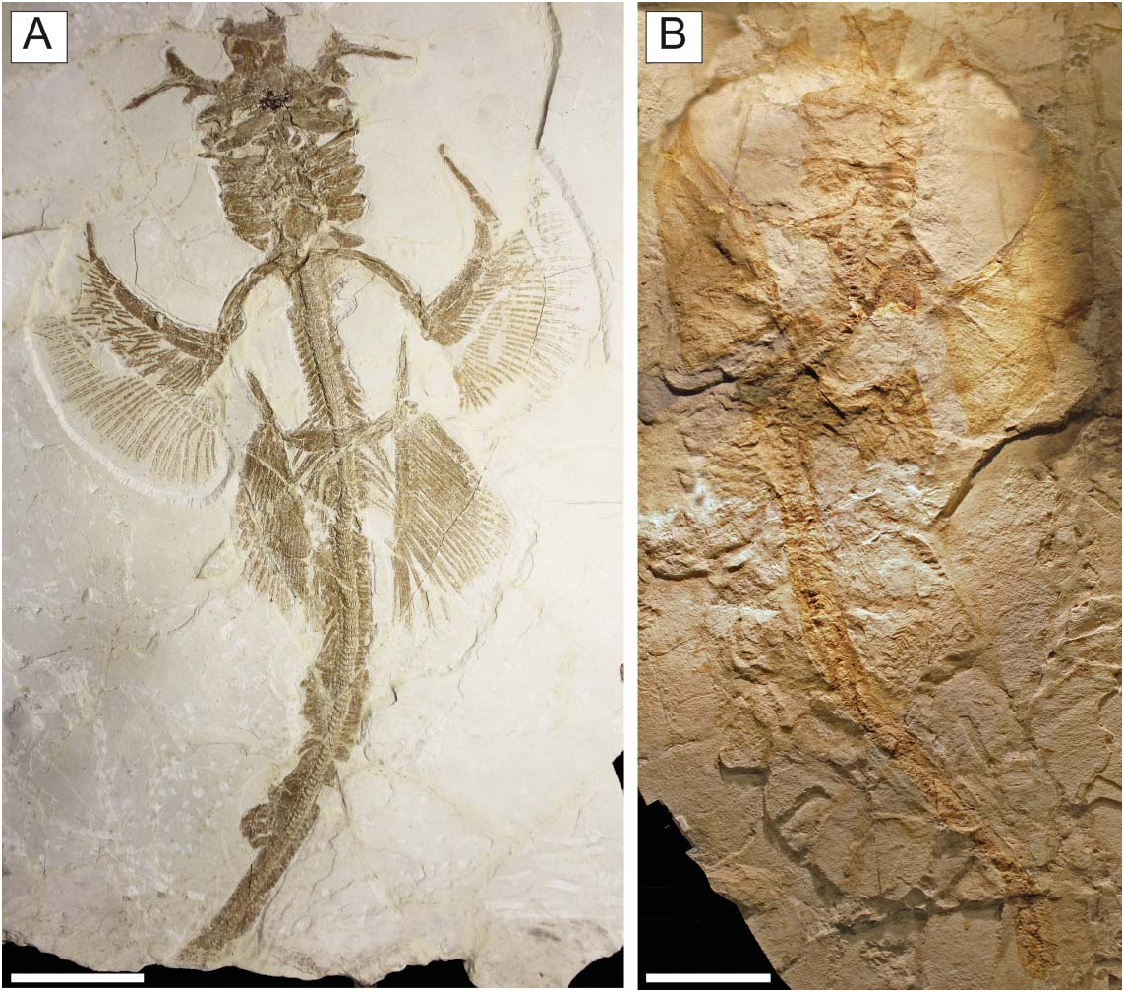
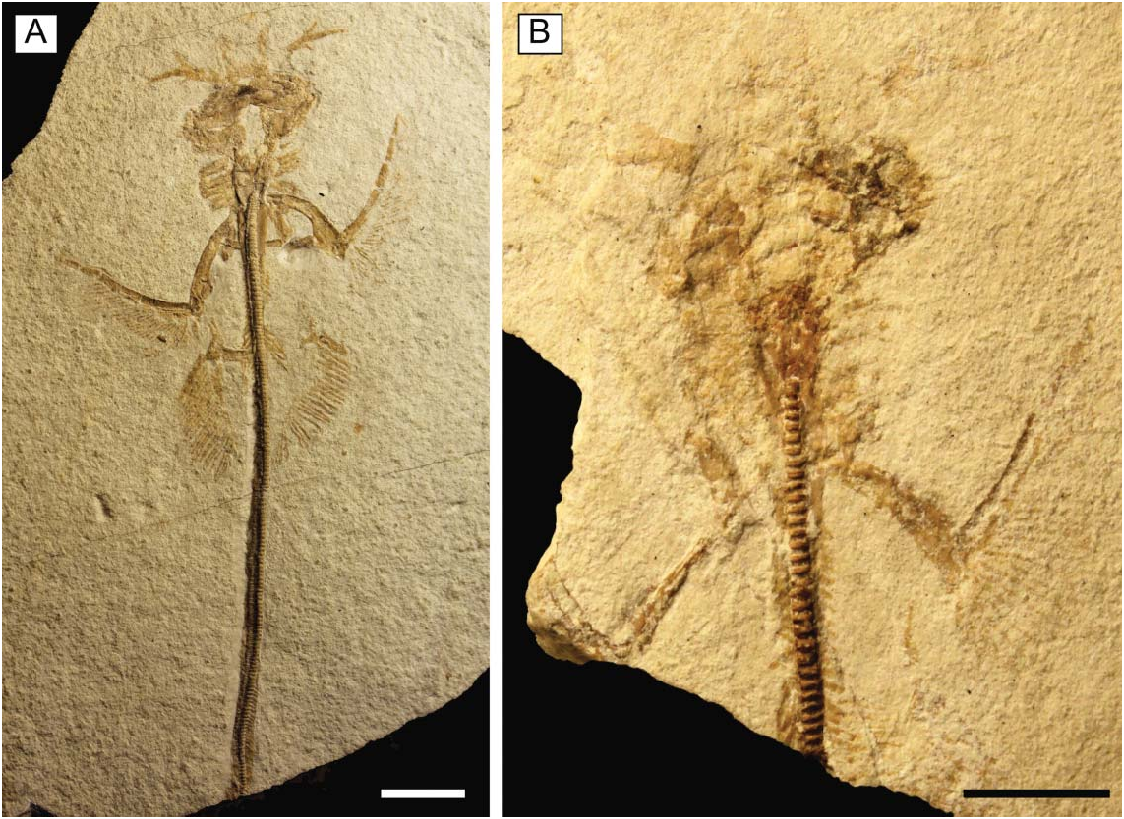
Referred material. MCSNV IG.VR.67290, nearly complete articulated skeleton in a single slab, 750.2 mm SL ( Fig. 3A View Figure 3 ); MCSNV IG.91128/9, partially complete articulated specimen in part and counterpart, 850.4 mm SL ( Fig. 3B View Figure 3 ); MCSNV IG.VR.91359, nearly complete articulated skeleton in a single slab, 99.0 mm SL ( Fig. 4A View Figure 4 ); MCSNV IG.135581, incomplete articulated specimen in a single slab, lacking part of the cranial and caudal regions ( Fig. 4B View Figure 4 ).
Type locality and horizon. Monte Postale site, Bolca Konservat-Lagerst¨atte, Italy; early Eocene, late Ypresian, middle Cuisian, SBZ 11, Alveolina dainelli Zone (see Papazzoni et al. 2017).
Diagnosis. Species of † Titanonarke characterized by the following combination of characters: subcircular disc length c. 44% SL and disc width c. 50% SL; greatly elongated precaudal tail of c. 60% SL; 153–155 vertebrae (27–30 trunk; 100–115 precaudal, 23–25 caudal); 15–16 tooth rows in upper jaw and 12–13 tooth rows in the lower jaw; c. 40 pectoral radials (12–16 propterygial, 9–12 mesopterygial, and 10–11 metapterygial); single-lobed pelvic fin with 21–24 basipterygial radials; first dorsal fin with 7–9 radials, and second dorsal fin with 6–7 radials; caudal fin with about 42 radials (20 dorsal and 22 ventral); width of pelvic fins about 50% of disc width; anterior pelvic fin margin length c. 24% of disc length.
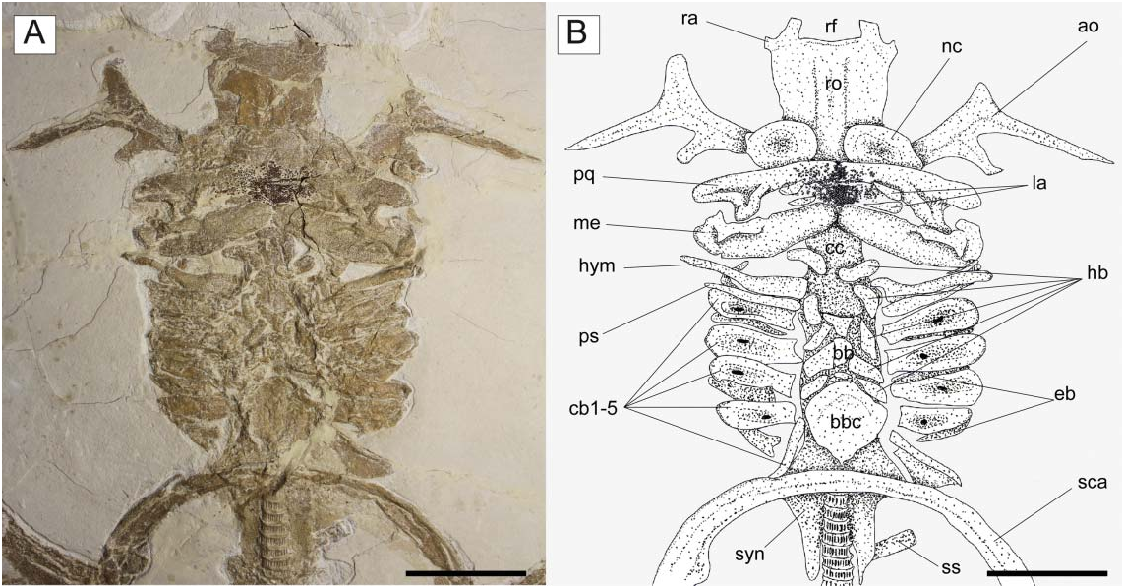
Description. Specimens examined comprise different ontogenetic stages and range from 99 to 850.4 mm SL, with the largest specimen reaching 925 mm TL. Measurements and counts for † Titanonarke molini are summarized in Tables 1 View Table 1 and 2 View Table 2 . Overall, the body is large and dorsoventrally compressed ( Figs 2 View Figure 2 , 3 View Figure 3 ). The disc is subcircular, slightly ovoid in outline, generally wider than long, and barely overlapping the pelvic fin origin. The largest width of the disc is slightly posterior to its midlength and its edges are continuously curved. The length and width of the disc are about 44% and 50% SL, respectively. The head is approximately 25% of SL and the preoral length is about 9% SL. The precaudal tail is elongated (about 60% SL). † Titanonarke molini has two dorsal fins; the predorsal distance at the first dorsal fin is about 62% SL, whereas the predorsal distance at the second dorsal fin is about 78% SL; the interdorsal distance is about 7% SL. The body is totally naked, lacking denticles and thorns. The skeleton is highly calcified and most of the skeletal elements, including parts of the rostral cartilage, jaws, hyomandibulae, antorbital cartilages, synarcual, and pectoral and pelvic girdles, show the typical prismatic calcification of elasmobranch fishes ( Dean & Summers 2006).
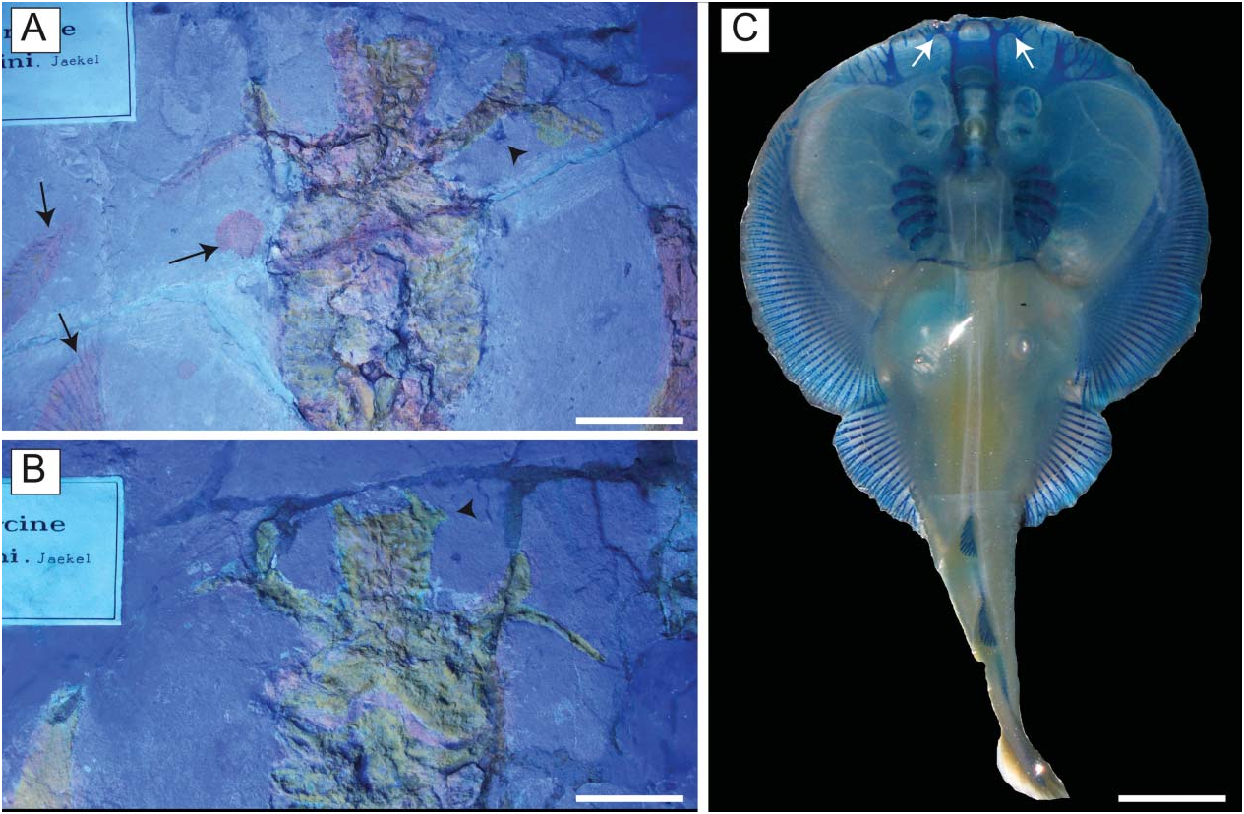
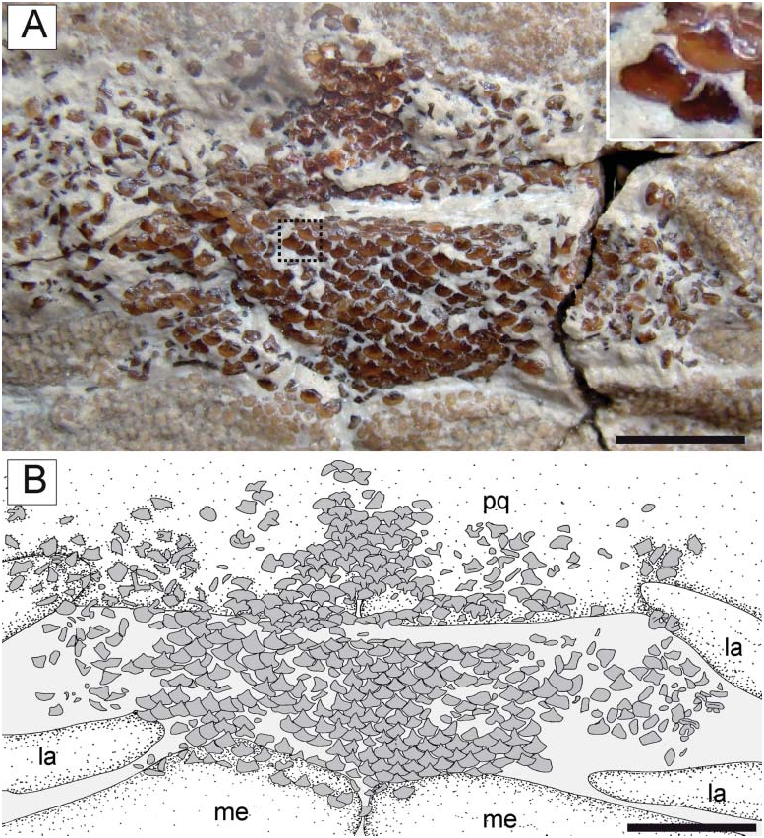
Chondrocranium. The rostral cartilage is expanded, dorsoventrally flattened and trough shaped ( Figs 5 View Figure 5 , 6 View Figure 6 ), resembling the typical condition of narcinids (Miyake et al. 1992). The rostrum is long, about one-half of the cranial length, wide anteriorly and tapering proximally towards the nasal capsules. The anterior margin of the rostral cartilage is concave, indicating the presence of a rostral fontanelle (= part of the precerebral fontanelle of Miyake et al. 1992), that is present also in Benthobatis , Diplobatis , Discopyge and Narcine ( Claeson 2014) and which also is supported by the phylogenetic analysis of this study (see below). Small lateral projections (= rostral appendices of Holmgren 1941; lateral rostral cartilage of Carvalho 1999) off of the rostral fontanelle were indicated in the holotype by Carvalho (2010). Due to the grout and/ or pigment used on the holotype MGP-PD 26275/6, UV light was not useful to distinguish further details of preserved tissues (see Fig. 7A, B View Figure 7 ). Ultraviolet light did highlight a possible lateral projection ( Fig. 7B View Figure 7 ), but we cannot discern whether it was branching and connected to the antorbital cartilage as in Narcine brasiliensis (Olfers, 1831) ( Fig. 7C View Figure 7 ). Evidence of a lateral projection off of the rostral fontanelle is, however, clearly present in MCSNV IG.VR.67290 ( Fig. 5 View Figure 5 ), but not in juvenile specimens of † Titanonarke that we examined, which thus resemble juveniles of Narcine brasiliensis (Miyake et al. 1992, fig. 14A). The connection of the rostral cartilage to the antorbital cartilage through the lateral projection was also detected in Diplobatis ( Fechhelm & McEachran 1984, fig. 5), Benthobatis (Rincon et al. 2001, fig. 7) and all narcinids in general ( Fechhelm & McEachran 1984, fig. 16; Carvalho 1999, 2010). This character, which is unique among torpediniforms, corroborates the monophyly and the systematic position of narcinids in this study. The juvenile specimen MCSNV IG.VR.91359 shows the early stage of the development of the rostral cartilage ( Fig. 6 View Figure 6 ), with at least one cartilaginous strip (= sensu Miyake et al. 1992) and two lateral rostral bars. The rostral cartilage of † Titanonarke lacks the basonasal fenestrae that characterize Diplobatis ( Fechhelm & McEachran 1984) . Although probably present on the dorsal surface of the chondrocranium, it is not possible to distinguish the presence of the anterior and frontoparietal fontanelle in the available material.
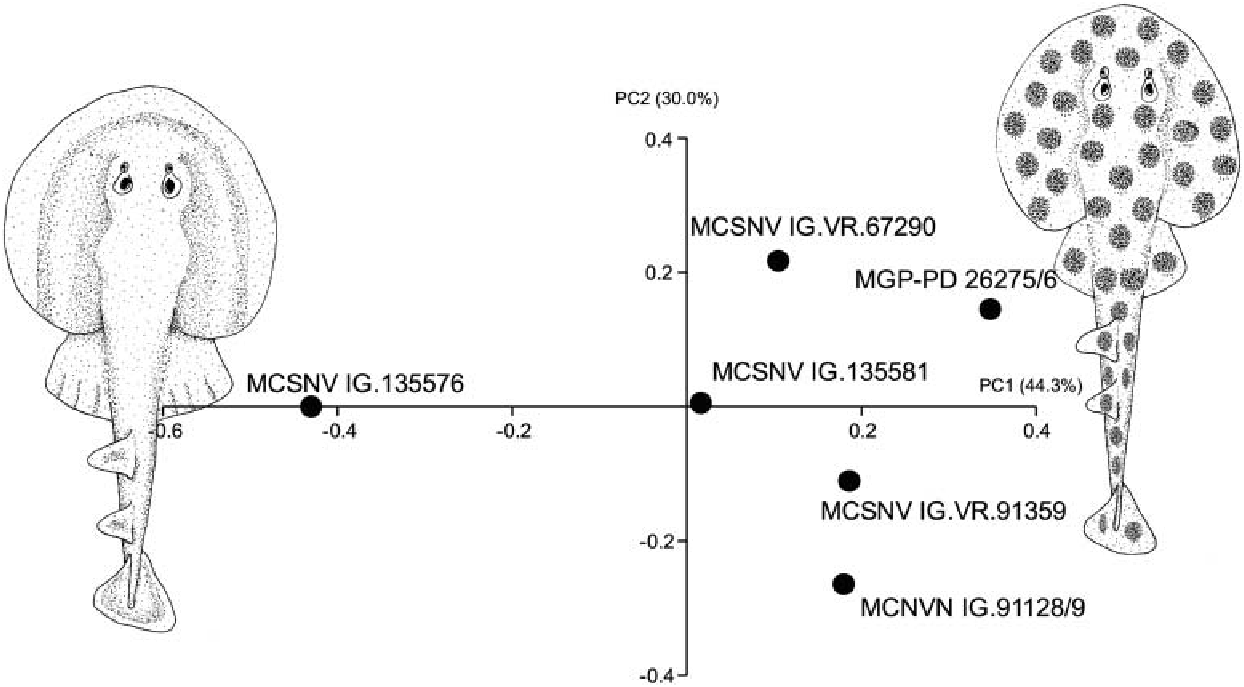
The antorbital cartilages in juveniles and adults are robust, well developed, directed anteriorly and broadly branching ( Figs 5 View Figure 5 , 6 View Figure 6 ). There are no distinct foramina on the anterior cartilages. Antorbitals are subdivided at about one-third of their length into two main branches; their stout bases articulate with the lateral aspect of the nasal capsules through an expanded posterior condyle. The distal end of the largest branch is broadly separated from the anterior extension of the propterygium. A third, smaller, posteriorly directed branch is located at midlength of the antorbital cartilages ( Figs 5 View Figure 5 , 6 View Figure 6 ). The absence of the posterior branch was considered diagnostic for † Titanonarke to the exclusion of Narcine and Discopyge by Carvalho (2010). However, the presence of this posterior branch in MCSNV IG.VR.67290, MCSNV IG. VR.91359, MCSNV IG.135581 and possibly, at least partially, in the holotype ( Figs 7A View Figure 7 ), suggests that it was not discernible in the specimens described by Carvalho (2010). Moreover, since osteological, morphometric and meristic features are not useful to separate juvenile specimens from the holotype, there is no reason to assign this smaller specimens to a new genus, if only based on the presence of this character. In fact, the PCA performed in this study on the entire morphological data set of standardized and log-transformed measurements and counts ( Fig. 8 View Figure 8 ) shows no remarkable separation of the juvenile specimens MCSNV IG.VR.91359 and MCSNV IG.135581 from the holotype and other referred material.
The nasal capsules are ovoid in shape, located at about midlength of the chondrocranium and projected laterally. The internasal plate between the two nasal capsules appears flat and narrow. As all specimens are preserved in dorsoventral view, it was not possible to detect the lateral aspect of the nasal capsules. The otic capsules and the posterior part of the chondrocranium are partially hidden by the jaws and hyoid arches. However, they appear to be approximately as wide as the widest part of the rostrum and much narrower than the nasal capsules. A large basicranial fenestra is recognizable in the juvenile MCSNV IG.VR.91359 ( Fig. 6 View Figure 6 ), which resembles in position and shape the condition shown in the early stages of chondrification of the otic region in Narcine brasiliensis (Miyake et al. 1992, fig. 14).
Jaws. Carvalho (2010) considered both palatoquadrate and Meckel’s cartilage of † T. molini broadly arched and not very stout when compared to those of other members of Narcinidae . Given that long, slender and curved jaws are diagnostic for the torpedinoids Torpedo and Hypnos whereas in narcinoid genera the jaws are short and stout ( Claeson 2014), the description of the jaws of † Titanonarke is further clarified here. The palatoquadrate of † Titanonarke is labiolingually compressed, narrower than the Meckel’s cartilage, and tapers towards the symphysis ( Fig. 5 View Figure 5 ). Like the palatoquadrate of Narcine brasiliensis (see Dean & Motta 2004a), the palatoquadrate of † T. molini possesses a strong condyle that articulates with the Meckel’s cartilage at the mandibular articular fossa sensu Dean & Motta (2004a). The Meckel’s cartilages are stout, flat and broad. There are two pairs of small, slender and subtriangular labial cartilages situated near the symphysis of the jaws, surrounding the tooth bands ( Fig. 5 View Figure 5 ). Combined, upper and lower labial cartilages are less than the length of the Meckel’s cartilage. The triangular element displaced to the corner of the left jaw joint in MCSNV IG.135576, and interpreted by Carvalho (2010, fig. 5b) as a labial cartilage, instead appears to be a dorsal flange of the sustentanculum of the Meckel’s cartilage. Both jaws are not fused medially.
Hyoid and gill arches. The exquisite preservation of MCSNV IG.VR.67290 allows a detailed description of most of the hyoid arch ( Fig. 5 View Figure 5 ). The hyomandibulae are narrow and elongate, slightly stout at the proximal base, and tapering distally towards their articulation with the Meckel’s cartilage. The dorsal and ventral pseudohyoids are long and slender, and located just posterior to the articulation of the hyomandibula with the otic region of the chondrocranium. † Titanonarke lacks ceratohyals, resembling the condition of Narcine and Discopyge among narcinoids (Miyake & McEachran 1991).
There are five pairs of ceratobranchials. The anterior four pairs are large, and have a central depression (or fossa) with a small fenestra for the insertion of the depressor muscles (see Carvalho & Śeret 2002). The fenestrae of † Titanonarke appear larger in small individuals ( Fig. 6 View Figure 6 ), as in Diplobatis (Miyake & McEachran 1991, fig. 6H; Claeson 2014, supplemental pl. 12). The fifth ceratobranchials are slender and posteriorly oriented, and articulate with the scapular process of the scapulocoracoid. The epibranchial elements are not clearly recognizable, being partially covered by the ceratohyals. At least three small basibranchials are recognizable. The basibranchial copula is large and rounded, with a small caudal tip or tab in its posterior margin. We counted five pairs of ovoid or beanlike hypobranchials. Hypobranchials are segmented, resembling the plesiomorphic condition of Benthobatis , Discopyge and Heteronarce among narcinoids (Miyake & McEachran 1991, fig. 6F, G; Claeson 2014, supplemental pl. 12). Pharyngobranchials, extrabranchials and branchial rays are not preserved in the available material.
Synarcual and vertebral column. The synarcual cartilage is strongly calcified in all mature specimens, though not in the embryo preserved inside the abdominal cavity of the holotype (see the section ‘Embryo’). In mature specimens the synarcual exhibits the typical prismatic tessellated cartilage found in elasmobranchs. The posteroventralmost portion of tessellated cartilage flanks several mineralized vertebral centra, which comprise densely packed areolar cartilage. Anteriorly, the synarcual cartilage contacts the occipital condyles of the chondrocranium via the occipital cotyles. The morphology of the anterior portion of the synarcual is difficult to discern, it being partially hidden by the hyoid arch (most of the specimens are ventrally exposed). Therefore, the position of the dorsal rim of the anterior neural canal opening (synarcual mouth) with respect to the occipital cotyle and the lips, as well as the shape of the ventral rim of the anterior neural canal opening (synarcual lip), are difficult to discern.
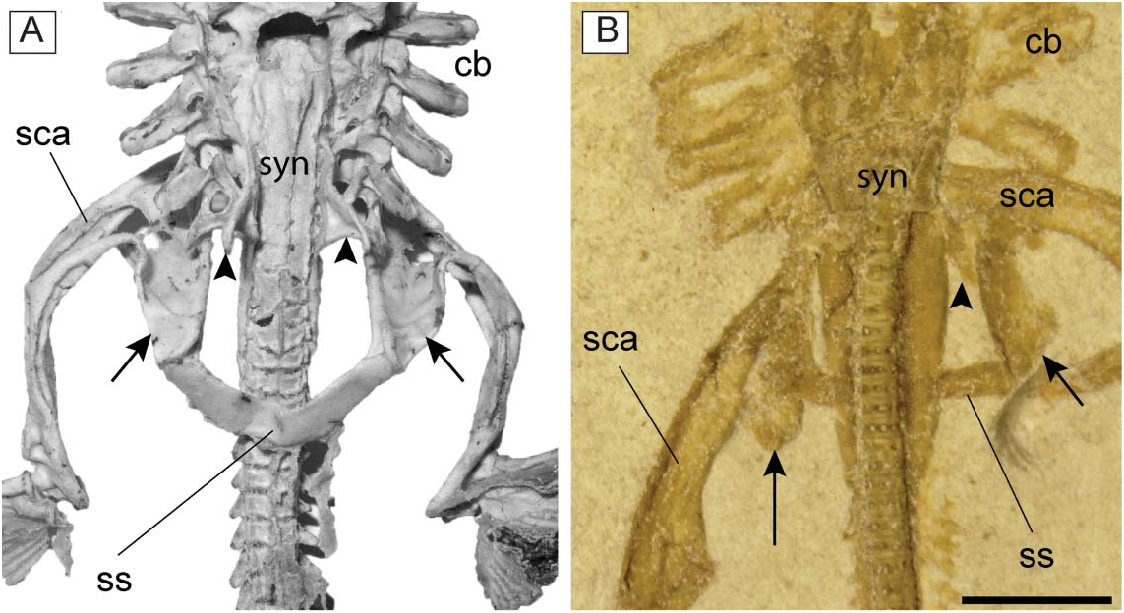
The synarcual possesses lateral stays, which are posteriorly displaced ( Fig. 9 View Figure 9 ) as in Benthobatis , Discopyge , Narcine , Heteronarce and Narke ( Claeson 2014) . The distal end of the lateral stays is apparently tab-like and their anterior margin forms an obtuse angle with the anterolateral margin of the synarcual. The long posterior flanges of the synarcual surround the anteriormost 8–12 free vertebral centra, and their posterior margins end posteriorly to the scapulocoracoid bar, resembling the condition of nonnarkid torpediniforms ( Compagno 1999). It is not possible to detect the number of fused vertebrae that constitute the synarcual, or the foramina.
The vertebral column of † T. molini consists of about 153–155 vertebral centra; of these, 27–30 are trunk centra (18–20% of total, from the first distinguishable centrum to the anterior margin of the puboischiadic bar), 100–115 are precaudals (65–75%, from the anterior margin of the puboischiadic bars to the upper origin of the caudal fin), and 23–25 are caudals (15–16%, from the upper caudal fin origin to the end of the series). The number of vertebrae is by far the largest compared to all living torpediniforms and can be considered an autapomorphic condition of † Titanonarke (see Table 2 View Table 2 ).
The vertebral centra are strongly calcified, subrectangular in shape and anteroposteriorly short. Large basiventral processes are visible along most of the vertebral centra, from the posterior tip of the synarcual to the caudal fin base. There are eight to 10 pairs of ribs articulating with centra posterior to the puboischiadic bar ( Fig. 11A View Figure 11 ). The low number of rib pairs is similar to that of Benthobatis , Discopyge , Diplobatis and Narcine and is considered a derived trait of the narcinids among torpediniforms (see the phylogenetic analysis in this study).
Paired fins and girdles. The scapulocoracoid is the largest element of the pectoral girdle. It is robust and strongly arched, and its anteriormost margin is situated between the basibranchial copula and the posterior tips of the synarcual flanges. The scapulocoracoid articulates anteriorly with the fifth pair of ceratobranchials. The suprascapulae are slender and fused medially, forming a slightly bowed bar in MCSNV IG.VR.91359 ( Fig. 6 View Figure 6 ). In MCSNV IG.VR67290 the suprascapula appears to be inclined with respect to the vertebral column, confirming the interpretation of a bowed shape ( Fig. 5 View Figure 5 ). The suprascapular antimere is longer than the scapular process of the scapulocoracoid, which is posteriorly directed as in Narcine ( Fig. 9 View Figure 9 ). The fusion of the suprascapular antimere with a visible suture is considered a synapomorphy of Torpediniformes by Claeson (2014). Although MCSNV IG.VR.67290 and MCSNV IG.VR.91359 preserve this skeletal element ( Figs 5 View Figure 5 , 6 View Figure 6 , 9 View Figure 9 ), it is not possible to describe a visible suture because both specimens are exposed ventrally and this area is obscured by the vertebral column. However, it is expected that † Titanonarke shares this character with all other electric rays.
The juvenile MCSNV IG.VR.91359 shows a weak taphonomic displacement of the suprascapulae with respect to the vertebral column ( Figs 6 View Figure 6 , 9 View Figure 9 ), which supports the hypothesis that the suprascapulae in torpediniforms are completely separate from the vertebral column and that the only connection between pectoral girdle and postcranium is through the fifth ceratobranchial ( Aschliman et al. 2012a; Claeson 2014). The same specimen shows that the suprascapular projection of † Titanonarke was lateral and that the suprascapula-scapulocoracoid articulation was loose and unforked.
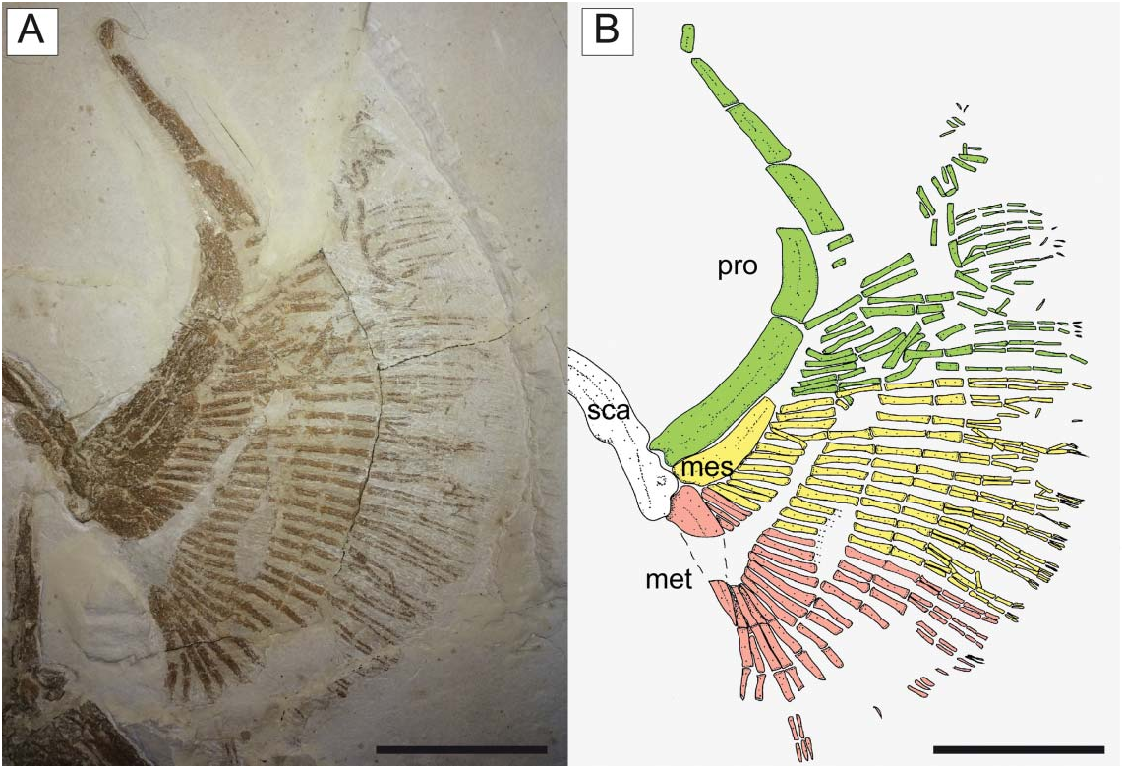
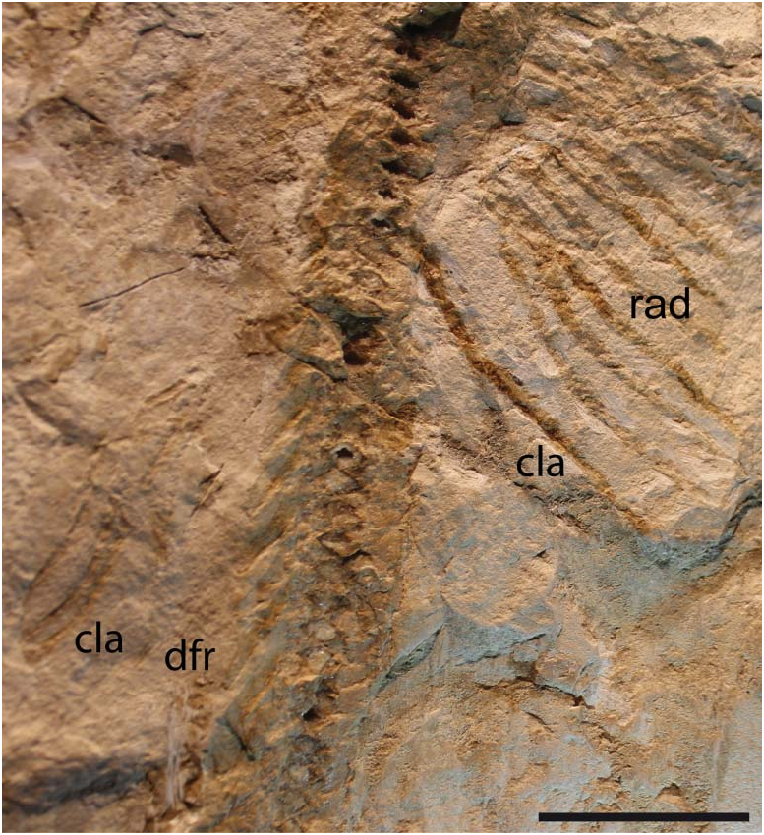
The propterygia are long and arched and extend well beyond the anterior margin of the scapulocoracoid ( Fig. 10 View Figure 10 ). They are composed of five or six propterygial segments. Two large voids, occupied in life by electric organs, are delimited by the propterygia, antorbital cartilages, hyoid archs and scapulocoracoids. The mesopterygium is flat, subtriangular in shape, and parallel and adjacent to the propterygium. The metapterygium is long and slender, is triangular in shape, and tapers posteriorly. The mesopterygium is shorter than the pro- and metapterygium in all specimens, although the apparent smaller size of the metapterygium in MCSNV IG.VR.67290 appears to be preservational (the metapterygium is weakly calcified in some narcinids; Carvalho & Śeret 2002) or an artefact of the glue used to join pieces of the slab during preparation. There are about 40 pectoral radials (12–16 propterygial, 9–12 mesopterygial and 10–12 metapterygial), which bifurcate twice before reaching the edge of the pectoral fin margin. Each radial is composed of four segments before the first bifurcation, and another four elements before the second bifurcation (at least 9–10 segments in total; Fig. 10 View Figure 10 ). The lower number of segments recognized by Carvalho (2010), and actually detected in some specimens, is probably related to the loss of distal elements due to taphonomic processes. The radials are covered with a continuous layer of small (less than 1 mm) tesserae, forming the so-called ‘crustal’ calcification that characterizes the radials of basalmost batoids having an axial-undulatory swimming style, including pristids and ‘rhinobatids’, other than torpedinids and narcinids (Schaefer & Summers 2005).
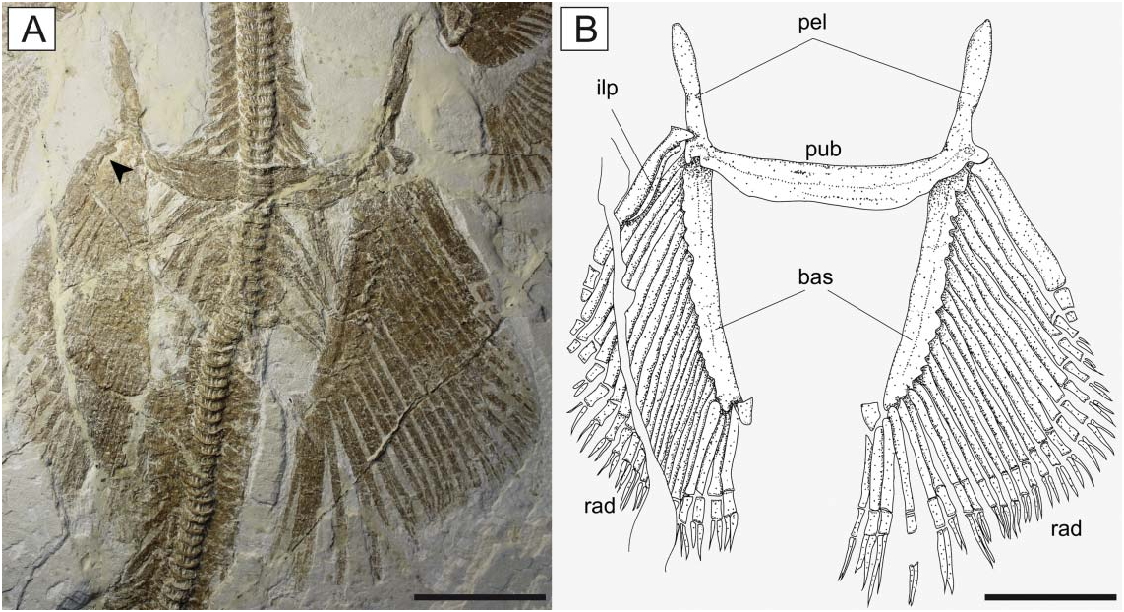
The pelvic fins ( Fig. 11 View Figure 11 ) are small and single-lobed, and their anterior margin is straight and barely overlapped by the posterior margin of the pectoral disc. The maximum width of the pelvic fins is about 50% of the pectoral disc width, whereas their anterior margin is about 24% of the disc length. The puboischiadic bar is robust and wide, with a slightly concave anterior margin. The presence of the puboischiadic foramina is difficult to detect. The prepelvic processes are long and straight, extending anteriorly almost to the level of the scapulocoracoid. They are wider distally than along the shaft, resembling the condition of all narcinids (Rincon et al. 2001, fig. 8; Fechhelm & McEachran 1984, figs 7, 16). The distal end of the prepelvic process was described as spatulate by Carvalho (2010). However, the margin of this structure, identified with an arrowhead on the holotypic specimen MGP-PD 26275/6 by Carvalho (2010, fig. 7), is not part of the prepelvic process. The iliac process, preserved only on the left side of MCSNV IG.VR.67290, appears short, stout and straight ( Fig. 11 View Figure 11 ) if compared to the long and curved iliac process of Narcine and Discopyge (Menni et al. 2008; Claeson 2014). The basipterygia are slightly longer than the puboischiadic bar, and have a slightly concave inner margin. Each basipterygium supports about 21–24 pelvic fin radials, the first of which is enlarged, articulates with the lateral node of the puboischiadic bar and supports the anterior margin of the pelvic fin. Each pelvic fin radial bifurcates distally once and is composed of three segments. A single individual ( MCSNV IG.91128/9) seems to show an elongate clasper, articulating with the distal tip of the basipterygium ( Fig. 12 View Figure 12 ). In length, the clasper appears to extend past the posterior tip of the pelvic fin lobe. However, it was not possible to analyse this structure in detail.
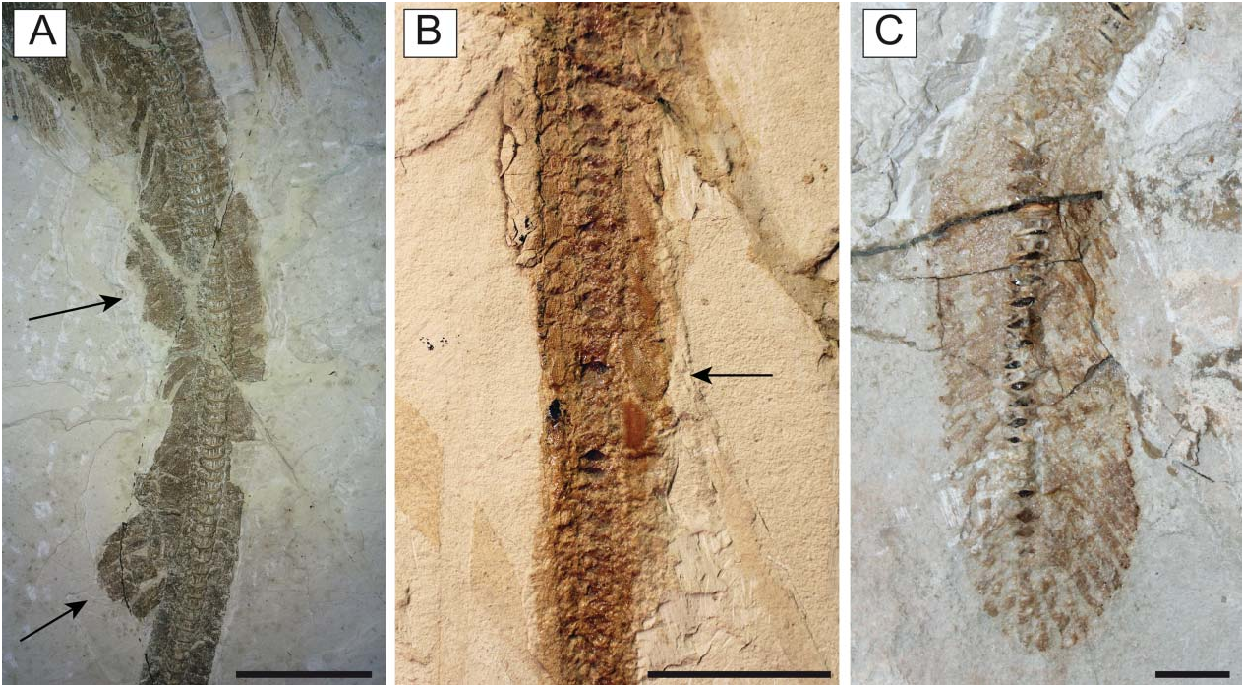
Median fins. There are two dorsal fins ( Fig. 13A View Figure 13 ). The first one originates at about 62% SL, is slightly larger than the second one and is supported by seven to nine radials. The second dorsal fin originates at about 78% SL and is supported by six or seven radials. The interdorsal distance measures about 7% SL. The obvious presence of two dorsal fins in MCSNV IG.VR.67290 supports the interpretation of Jaekel (1894) and Cappetta (2012) regarding the presence of at least one dorsal fin in the holotype MGP-PD 26275/6 ( Fig. 13B View Figure 13 ). The inadequate preservation of the dorsal fins in the historical material prevented their recognition by Carvalho (2010), who erroneously regarded their absence as diagnostic for † Titanonarke . About 42 radials support the caudal fin, of which about 20 are dorsal and 22 ventral ( Fig. 13C View Figure 13 ).
Dentition. The teeth of † Titanonarke are arranged in tooth bands medially across the jaw symphyses, forming a tessellated pavement ( Fig. 14 View Figure 14 ). The lower tooth band is wider than the upper one. It is not possible to detect the tooth formula, but the teeth of † T. molini appear to be arranged in at least 12–13 rows in the upper jaw and 15– 16 rows in the lower jaw, counted on symphyseal tooth series. The dentition is gradient monognatic heterodont with lateral and posterior tooth crowns becoming slightly lower. However, both upper and lower jaws show very little heterodonty. Sexual heterodonty (studied via the analysis of the specimens with and without claspers) appears to be absent. It is not possible to detect any ontogenetic heterodonty due to the poor-quality preservation of this region in juveniles.
The tooth morphology is generally consistent with that of Narcine (see Herman et al. 2002, pls 10, 11). A single narrow, high and subtriangular cusp is present in each tooth. There are no accessory lateral cusplets. The crown base is broad and subcircular, and wider than the cusp length. The width of the cusp is less than half the length of the cutting edges. Cutting edges are blade-like. Lingual and labial ornamentations are absent and the tooth crown is completely smooth. Some teeth display a high and narrow root.
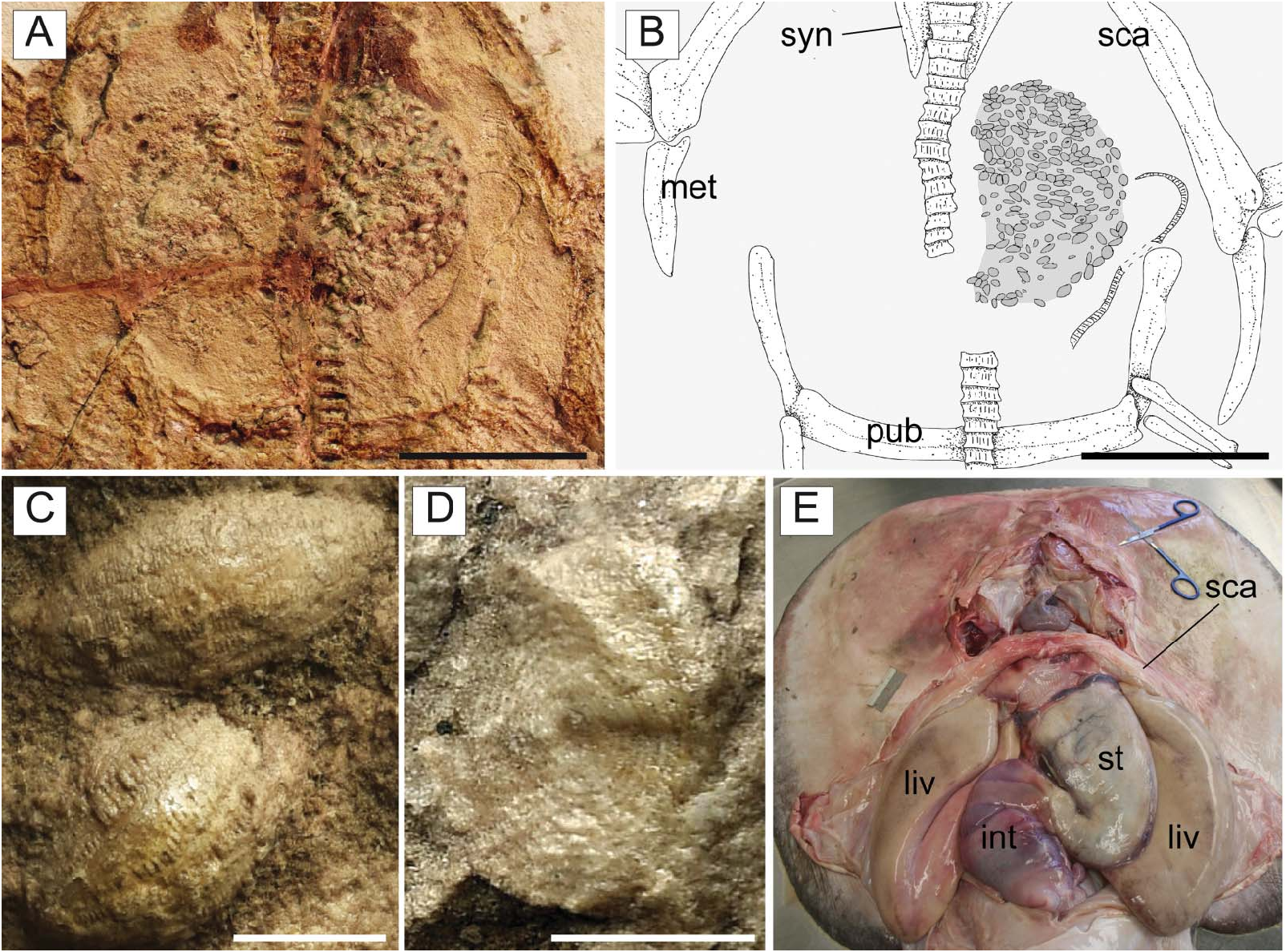
Gut contents. The holotype of † T. molini ( MGP-PD 26275/6) shows abdominal contents consisting of a pelletlike accumulation of hundreds of specimens of † Alveolina ( Fig. 15A–D View Figure 15 ), which is the most common foraminiferan genus in the Monte Postale sediments (Papazzoni et al. 2017). The individual foraminifera are grouped together and closely packed, forming an accumulation, which is ovoid in outline and anteroposteriorly elongate, measuring about 80.5 and 41.8 mm in length and width, respectively. The exceptionally preserved gut contents show little evidence of digestion, suggesting that consumption occurred shortly before the numbfish’s death. The accumulation is almost totally preserved in the abdominal cavity between pectoral and pelvic girdles, on one side of the vertebral axis, and just posteriorly to the flanges of the synarcual cartilage. This position is exactly comparable to that occupied by the gut-intestine tract in living electric rays ( Fig. 15E View Figure 15 ), and in narcinids in particular (see Marinsek et al. 2017, fig. 1). Moreover, the general shape of the accumulation resembles fossilized gastric contents detected in other elasmobranchs (e.g. Hovestadt & Hovestadt-Euler 2002; Amalfitano et al. 2017). Additionally, there is no evidence of skeletal dispersal due to currents or bioturbation. Thus, we conclude that the accumulation is not the result of processes related to bottom tractive currents or bioturbation that sometimes can result in the accumulation of such elements (see Sch¨afer 1972). Consequently, this accumulation of foraminifera unquestionably represents gut contents.
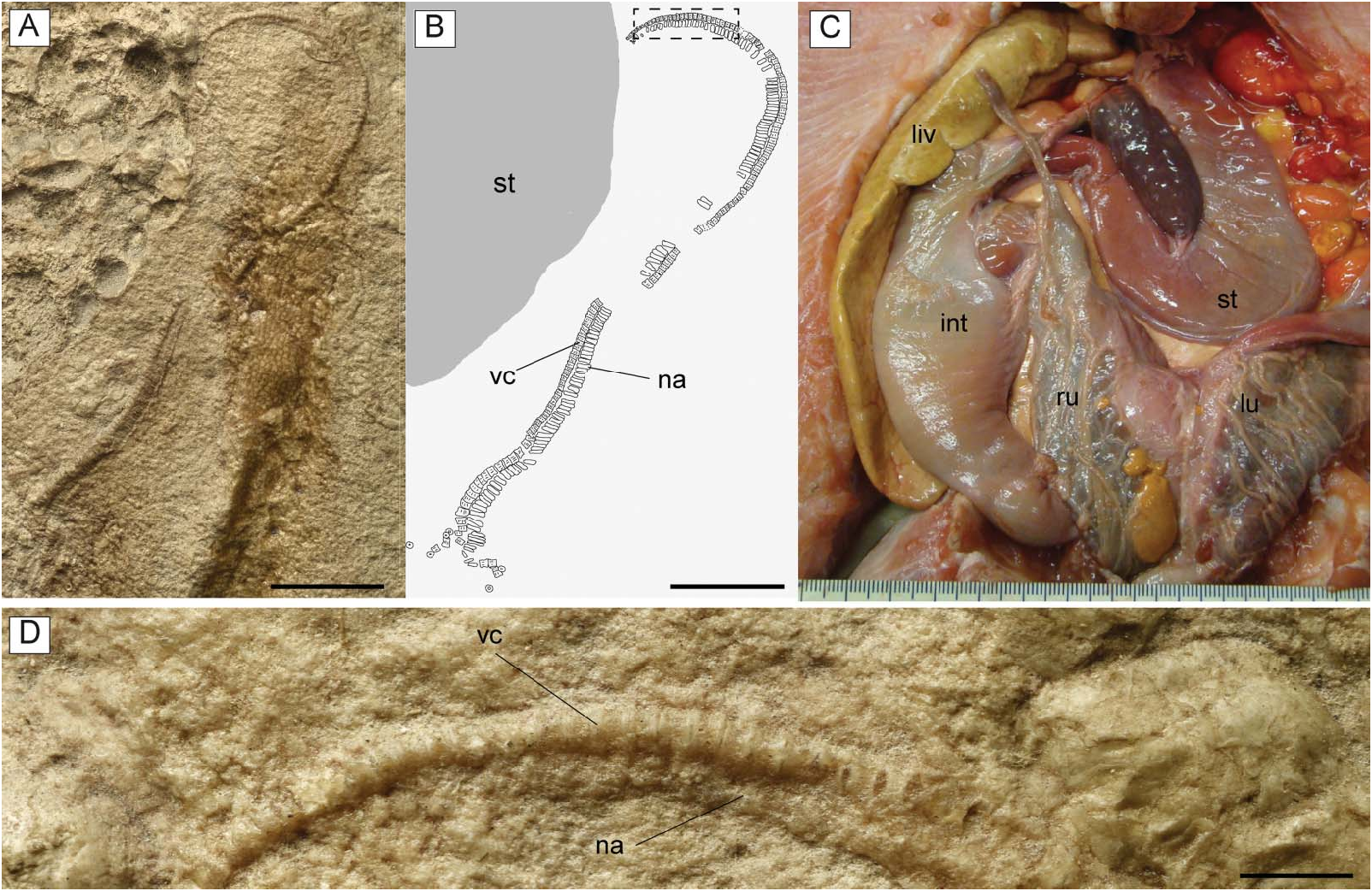
Embryo. MGP-UP 26275/6 also shows a unique partially developed embryo whose length is estimated to range between 50 and 60 mm ( Fig. 16 View Figure 16 ), about 6–7% of the adult size. The embryo comprises an almost complete vertebral column having about 150 vertebrae, whose number is perfectly comparable to that of an adult individual of † T. molini . The vertebral column is partially disarticulated, with some elements scattered from their original position. Each vertebra consists of a vertebral centrum and associated neural arch. There is no trace of the synarcual cartilage. This is consistent with the pattern of mineralization present in extant developing batoid embryos (e.g. Myliobatis , Pristis , Raja ; KMC pers. obs.), where the areolar cartilage of the free vertebrae is well mineralized earlier than the tessellated cartilage of the synarcual. The vertebral column is the only fossilized structure in this embryo. There are no cranial or girdle skeletal elements preserved. This condition is consistent with the late phases of skeletogenesis in elasmobranch fishes in which the mineralization of the cartilages involves only teeth, dermal denticles, vertebral centra and neural arches in very early stages (see e.g. Eames et al. 2007; Enault et al. 2016). However, teeth are not clearly recognizable in the embryo, whereas dermal denticles are expected to be absent, as in all torpediniforms.
The embryo is totally preserved in the abdominal cavity between the pectoral and pelvic girdle, on the left side of the vertebral axis, and just next to the stomach (see also Fig. 15 View Figure 15 ). This position is totally comparable to that occupied by the left uterus in fossil ( Carvalho et al. 2004, figs 2 and 13) and living batoids ( Fig. 16C View Figure 16 ; but see also Spieler et al. 2013, fig. 6), and in narcinids in particular (see Nair & Soundararajan 1973, fig. 1; Devadoss 1998, fig. 5). The embryo lies externally to the stomach (whose outline is clearly delimited by its contents of larger foraminifera; see Fig. 16A, B View Figure 16 ) therefore excluding the hypothesis of a possible ingested prey. The absence of traces of egg case surrounding the embryo suggests that the reproductive mode of † T. molini was viviparous (probably yolk-sac), a condition that resembles that of most living batoids ( Hamlett & Koob 1999; Kriwet et al. 2009), and narcinids in particular ( Hoar & Randall 1988; Bruton 1990; Rincon 1997; McEachran & Carvalho 2002; Last et al. 2016), and is considered plesiomorphic in batoids ( Cole 2010). Finally, although the general morphology, size and position of the embryo also resemble those already detected in fossil sharks (e.g. Hovestadt & Hovestadt-Euler 2010; Hovestadt et al. 2010) and extinct freshwater stingrays ( Carvalho et al. 2004), the specimen described herein unquestionably represents to our knowledge the first occurrence of a fossilized embryo in situ in marine batoid fishes.
Parasites. The examination of the historical material also has shown that fossilized crustacean isopods are strictly associated with the body of two individuals of both species of † Titanonarke . Individual fishes appear to be infested by two to four isopods each, the analysis of which is beyond the scope of this paper and will be provided in a separate study (Robin et al. in prep.).
| IG |
Institute of Geology |
| UV |
Departamento de Biologia de la Universidad del Valle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |