Sylvicanthon mayri, Cupello & Vaz-De, 2018
|
publication ID |
https://doi.org/ 10.5852/ejt.2018.467 |
|
publication LSID |
lsid:zoobank.org:pub:8D27AAB8-B7F2-424C-B1A6-66FEFA66EDFF |
|
DOI |
https://doi.org/10.5281/zenodo.3846329 |
|
persistent identifier |
https://treatment.plazi.org/id/260557DD-3E56-47DE-94AC-6F8DD638C039 |
|
taxon LSID |
lsid:zoobank.org:act:260557DD-3E56-47DE-94AC-6F8DD638C039 |
|
treatment provided by |
Valdenar |
|
scientific name |
Sylvicanthon mayri |
| status |
sp. nov. |
Sylvicanthon mayri View in CoL sp. nov.
urn:lsid:zoobank.org:act:260557DD-3E56-47DE-94AC-6F8DD638C039
Figs 13D View Fig , 16B View Fig , 19E View Fig , 20 View Fig , 41 View Fig , 43C, F View Fig , 44 View Fig A–B
Etymology
A tribute to the German-American ornithologist, evolutionary theorist, philosopher and historian of biology Ernst Mayr (1904–2005), author of some of the major classics in evolutionary biology and, in the first author’s view, one of the greatest and most versatile biologists of the 20 th century. In special recognition of the immense intellectual influence he had (and continues to have) on MC’s formation and in his choice to pursue a career as a systematist. Haffer (2007) presented the most complete biography of Ernst Mayr to date. The specific name is a noun in the genitive case.
Material examined
Holotype
BRAZIL: ♂, Acre, Senador Guiomard, Fazenda Experimental Catuaba , 10º04′ S, 67º37′ W (see comments below) (“ BRASIL: AC. Rio Branco / Faz. Catuaba II - 1997 / F.Z. Vaz-de-Mello leg.”), genital capsule extracted and glued to a point card ( CEMT).
GoogleMapsParatypes (1 ♂, 2 ♀♀)
BRAZIL: Amazonas: 1 ♀, São Paulo de Olivença, Jun.– Jul. 1883, M. de Mathan leg. ( CEMT).
COLOMBIA: Meta: 1 ♂ (dissected), “ 33 km E Villavicencio”, 2–4 Mar. 1972, dung trap, S. and J. Peck leg. ( CMNC).
PERU: Madre De Dios: 1 ♀, Tambopata, Puerto Maldonado, 12º21′19″ S, 69º01′48″ W, 221 m, 26–27 Mar. 2009, L. Figueroa leg. ( MUSM).
Description
COLOURATION. Head, pronotum, elytra, metaventrite, and pygidium dark green or dark blue. Meso- and metafemora reddish-brown; occasionally, with greenish reflections.
HEAD. Tegument shiny, with weak alveolar microsculpture and covered by dense well-marked micropunctation, which is almost imperceptible or even absent at apex of clypeus. Clypeus with two apical teeth obtuse only and only slightly separated from one another; with single transverse row of very short setae covering base of both teeth. Genae with small denticle immediately behind clypealgenal juncture. Posterior edge of head unmargined between eyes or with very fine, almost imperceptible interrupted line at centre.
THORAX. Pronotoum with shiny tegument and with dense micropunctation at centre; towards the sides, micropunctation progressively less well-marked, but always present, although occasionally almost imperceptible; alveolar microsculpture between micropunctures present only on anterolateral angles and in narrow strip on lateral margins; at centre, tegument between micropunctures smooth or with very subtle microsculpture (as on elytra). Posterior edge with fine transverse line at centre (usually extending up to second elytral stria) which occasionally may be difficult to see. Hypomeral cavity with some long yellowish setae at centre; external margin with slight, almost inconspicuous tubercle. Metaventrite glabrous at centre; sides with few sparse setae near anterior margin of metacoxae ( Fig. 7B View Fig ); posterior region of metaventrite with narrow transverse strip of tegument with distinct rivose microsculpture; centre and posterior region with dense micropunctation and without microsculpture.
LEGS.Ventral surface of all femora and tibiae bright.Profemora with tegument with sparse micropunctation and without microsculpture at anterior half, and with strong rivose microsculpture at posterior half. Protibiae narrow and with distinct expansion on the internal edge; at their apical third, external edge with three small acute teeth, the two most apical ones subequal in length and larger than the basal ( Fig. 11E View Fig ). Mesofemora margined anteriorly only at their basal half; unmargined portion of anterior edge covered by row of very short setae; posterior margin absent; tegument smooth and with sparse micropunctation, except for anterior half, which has rivose microsculpture. Metafemora margined only anteriorly, posterior margin absent; apical third of anterior edge covered by row of setae; tegument with rivose microsculpture at anterior half, and smooth with sparse micropunctation at posterior half; base with very short, ill-delimitated or even almost totally absent coarse punctures ( Fig. 13D View Fig ). Metatarsomeres II and V subequal in length and longer than the others; metatarsomere IV shorter than the others.
ELYTRA. With only six or seven narrow visible striae: the first three striae well marked, finely carinulate, and widened at base; striae IV–VII progressively more effaced and interrupted; all striae lack carinulae before reaching apex of elytra, where they are marked only by microsculpture or are completely indistinct; humeral carina absent. Tegument of interstriae shiny, with very diffuse microsculpture (never in a well-delimitated alveolar pattern as in S. furvus ) and with very dense micropunctation.
ABDOMEN. Tegument of ventrites I–V with strong rivose microsculpture; ventrite VI smooth at centre and with weaker rivose microsculpture on sides. Pygidium with shiny tegument, without microsculpture, and with dense micropunctation.
AEDEAGUS. Parameres much longer than half-length of phallobase, and without noticeable asymmetry, both external faces flat. In lateral view, parameres with apex widely bifurcate, with inferior branch of bifurcation distinctly projected and divergent from superior branch; without ventral keel or notch ( Figs 19E View Fig , 44 View Fig A–B).
SEXUAL DIMORPHISM. Males: Protibial spur broad and bifid, with external projection spiniform and not much longer than internal projection, which is bent and widened ( Fig. 15N View Fig ). Abdomen without lateral foveae. Ventrite VI strongly narrowed at centre due to distinct emargination on posterior edge; anterior edge slightly covered by weak medial flange of posterior edge of ventrite V. Pygidium very long (length between 1.2 and 1.1 mm) and convex; apical margin of pygidium much wider than lateral margins. Females: Protibial spur spiniform.Abdomen with three pairs of very shallow transverse foveae located between ventrites I–II, II–III, and III–IV, respectively; foveae not margined by row of long setae ( Fig. 16B View Fig ). Ventrite VI wide at centre, posterior edge straight, without emargination; anterior edge subtly covered by medial flange of posterior edge of ventrite V. Pygidium shorter (between 0.9 and 0.8 mm) and flat; apical margin of pygidium only slightly wider than lateral margins.
Measurements
Males (N = 2). TL: AV: 7.0 ± 0.35; MX: 7.3; MN: 6.8. EW: AV: 5.0 ± 0.21; MX: 5.2; MN: 4.9. PrL: AV: 2.3 ± 0.07 MX: 2.4; MN: 2.3. PrW: AV: 4.4 ± 0.21; MX: 4.6; MN: 4.3. PgL: AV: 1.15 ± 0.07; MX: 1.2; MN: 1.1. PgW: AV: 2.2 ± 0.14; MX: 2.3; MN: 2.1.
Females (N = 2). TL: AV: 6.75 ± 0.07; MX: 6.8; MN: 6.7. EW: AV: 4.8 ± 0.35; MX: 5.1; MN: 4.6. PrL: AV: 2.05 ± 0.07; MX: 2.1; MN: 2.0. PrW: AV: 4.15 ± 0.21; MX: 4.3; MN: 4.0. PgL: AV: 0.85 ± 0.07; MX: 0.8; MN: 0.9. PgW: AV: 2.05 ± 0.07; MX: 2.1; MN: 2.0.
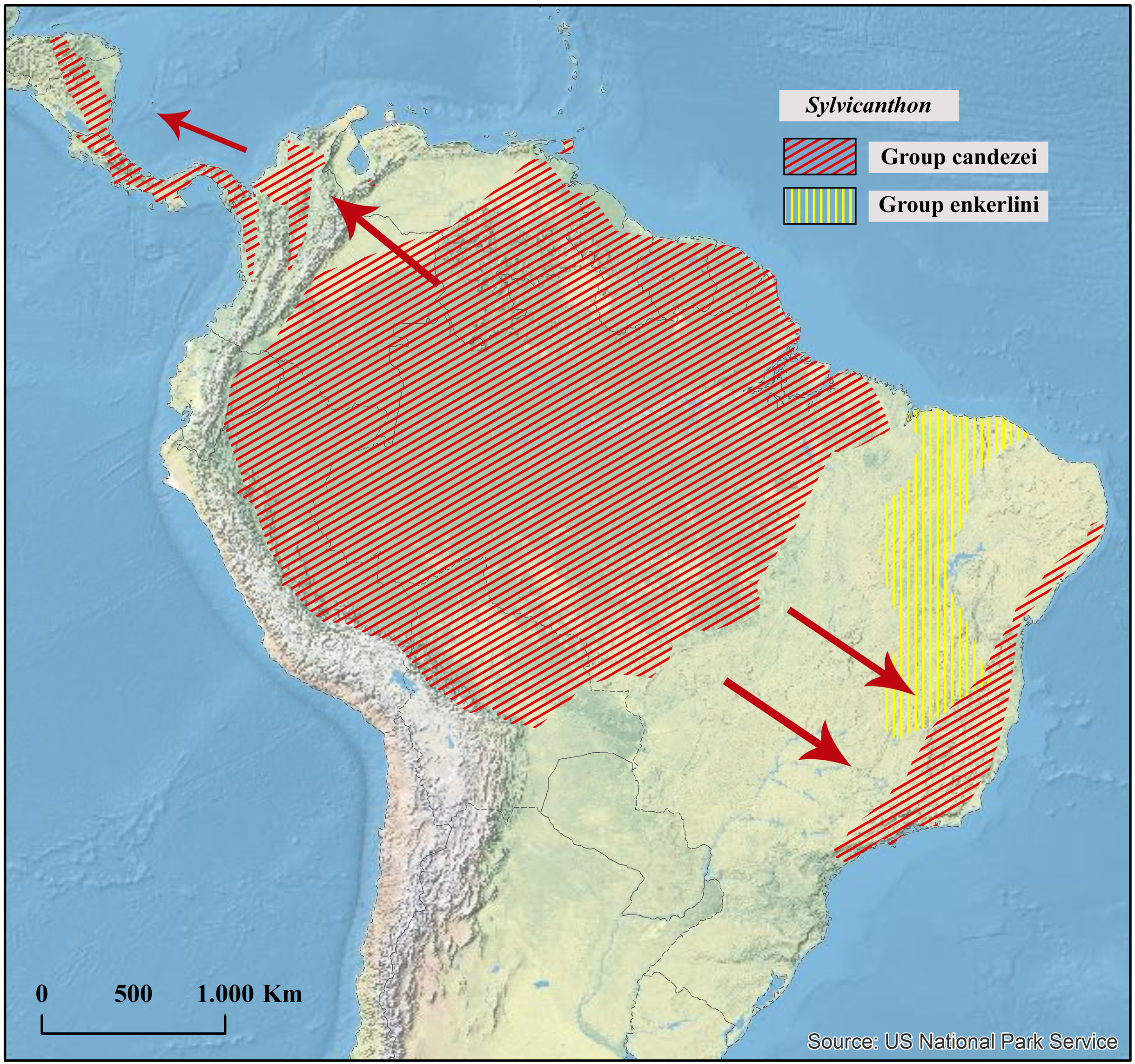
Geographical distribution
Western Amazonia, in Colombia, Brazil, and Peru.
Ecoregions
Guianan Moist Forests, Marajó Varzea, Japurá-Solimões-Negro Moist Forests.
Collecting sites ( Fig. 41 View Fig )
COLOMBIA. Meta: Villavicencio.
BRAZIL. Acre: Senador Guiomard. Amazonas: São Paulo de Olivença.
PERU. Madre de Dios: Tambopata (Puerto Maldonado).
Intraspecific variation and taxonomic discussion
Of all the species of Sylvicanthon , S. mayri sp. nov. is certainly the most enigmatic, with only four known individuals in collections, two males and two females. What we know about its distribution, for instance, is clearly fragmentary. The four known localities lie in western Amazonia and, together, they form a longitudinal arc with almost 2000 km from one end to the other (namely, Villavicencio, Colombia, in the north, and Puerto Maldonado, Peru, in the south) ( Fig. 41 View Fig ). Some questions can be raised from this observation: does S. mayri sp. nov. also occur in the interior of Peru and in Ecuador or northern Bolivia? Is its distribution extended eastwards into the lowlands of the Brazilian Amazonia? Does this species also occur across the eastern slopes of the Andes in Peru and Ecuador as it does in Colombia? These questions will only be answered when a larger number of dung beetle collections are made throughout the Amazon Basin, especially in the state of Amazonas ( Brazil), from where very little Sylvicanthon material is known (mostly from the capital, Manaus).
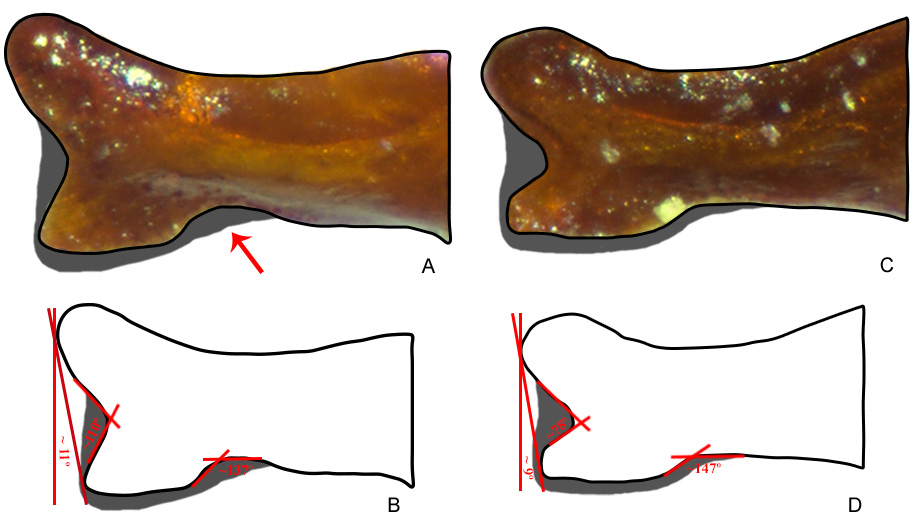
The morphological differences with the other two species of the furvus subgroup to which S. mayri sp. nov. is most similar ( S. furvus and S. monnei sp. nov.) are very clear even from the small number of specimens we currently have on hand. Sylvicanthon furvus , in general, is distinguished from S. mayri sp. nov. in presenting a very strong microsculpture throughout the body tegument. This is very clear especially on the meso- and metafemora (which are almost entirely smooth, except in the apical areas of rivose microsculpture in S. mayri sp. nov., while they are totally covered by rivose microsculpture in S. furvus ), and elytra (which have a diffuse microsculpture in S. mayri sp. nov., while they have a strong alveolar microsculpture obliterating the micropunctation in S. furvus ). From S. monnei sp. nov., S. mayri sp. nov. is different mainly in the shape of parameres (with a strongly bifurcate apex, with its inferior branch distinctly divergent from the superior one, in S. mayri sp. nov. ( Fig. 44 View Fig A–B), and apical bifurcation branches only weakly divergent and with inferior branch only little projected and straight in S. monnei sp. nov. ( Fig. 44 View Fig C–D)). Lastly, the coarse punctation at the base of metafemora is distinct between S. mayri sp. nov., on the one side, and S. furvus and S. monnei sp. nov., on the other: in the two latter species, those punctures are clearly impressed and are elongated ( Fig. 13C, E View Fig ), while they are very fine and almost indistinguishable from the micropunctation in S. mayri sp. nov. ( Fig. 13D View Fig ). For a more detailed comparison between these three species and also with S. obscurus , see the discussions of S. furvus , S. obscurus , and Table 5.
Comments
The holotype of Sylvicanthon mayri sp. nov. is part of a large series of dung beetles collected in 1997 by the second author in the Brazilian state of Acre (some of the results of those collections were published in Vaz-de-Mello 1999). The Fazenda Experimental Catuaba , research base administered by the Federal University of Acre, contrary to what is said on the holotype’s precedence label, is not located at the limits of the municipality of Rio Branco, but rather it is in the municipality of Senador Guiomard, about 25 km from the centre of Rio Branco. We revised for this work a great volume of envelopes with material collected by FZVM at Fazenda Catuaba and other nearby localities, but we failed to find any other specimens of S. mayri sp. nov. As argued earlier in this monograph, this fact should reflect the low population density the species of the furvus subgroup naturally have (see the discussion on S. furvus ).
Natural history
The only specimen of S. mayri sp. nov. with known food habit data is the paratype from Villavicencio, which was collected in a pitfall trap baited with dung. The collecting months accurately reported were February and March, with the period June─July being the date reported for the São Paulo de Olivença specimen. Nothing more is known about the biology of S. mayri sp. nov.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Scarabaeinae |
|
Tribe |
Deltochilini |
|
Genus |