Setosella alfioi, Rosso & Martino & Gerovasileiou, 2020
|
publication ID |
https://doi.org/10.11646/zootaxa.4728.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:3E4C6C25-3630-4842-A776-F87CF2E693FD |
|
persistent identifier |
https://treatment.plazi.org/id/0C3887F8-FF8B-FFC4-FF72-FF61ED797902 |
|
treatment provided by |
Plazi |
|
scientific name |
Setosella alfioi |
| status |
sp. nov. |
Setosella alfioi n. sp.
( Figs 18–21 View FIGURE 18 View FIGURE 19 View FIGURE 20 View FIGURE 21 ; Tables 1 View TABLE 1 , 2 View TABLE 2 , 5 View TABLE 5 )
? Setosella vulnerata: d’Hondt, 1981 , 36.
Setosella vulnerata: Di Geronimo et al., 1997 View in CoL : table 3 (pars).
Setosella folini: Di Geronimo et al., 2001: p. 283 View in CoL , table 3, pl. 2, fig. 2; Mastrototaro et al., 2010: p. 423, 425, table 2.
Type material. Holotype PMC. B26a. 30.6.2018, EOCUMM 1995, 27B, 1096 m depth, one dead colony fragment including five zooids and a half, mounted on a stub . Paratypes PMC. B26b. 30.6.2018, EOCUMM 1995, sample 27B, two dead fragments including one and two zooids and a half, respectively; sample 15, 1521 m depth, two dead fragments including one and three zooids respectively; sample 26, 945 m depth, one fragment including three zooids and a half. Off Eolian Islands, SE Tyrrhenian Sea
.
Additional material. PMC. Rosso Collection I.H. B-46a, APLABES cruise, Santa Maria di Leuca, off S Apulia, NE Ionian Sea; AP 1, 513 m depth, one living colony fragment including five zooids; AP 29, 790 m depth, one living colony fragment including 1–2 zooids. PMC. Rosso Collection I.Pl. B-46b, Pleistocene: Archi section, sample 6 (two isolated zooids), sample 12 (one zooid).
Diagnosis. Colony seemingly free-living, scorpioid, uniserial, starting from a proximally tapering zooid. Autozooids budding in a clockwise, elicoidal pattern, asymmetrical. Cryptocyst smooth. Opesia transversely D-shaped with an arched distal shelf formed by granulations, the two external the most prominent. Opesiules paired, asymmetrical, subcircular to elongate with denticles in the inner margin. Vibracula budded distally to each autozooid with flared rostrum, acorn-shaped opesia and symmetrical condyles. Terminal ovicell produced by a shorter maternal autozooid, elongate; ectooecium smooth, marked by a flam-shaped line and with a medial window exposing the smooth endooecium.
Etymology. Named after Alfio Viola (University of Catania), expert SEM technician, for his help in imaging some of the bryozoan specimens studied here and numerous others.
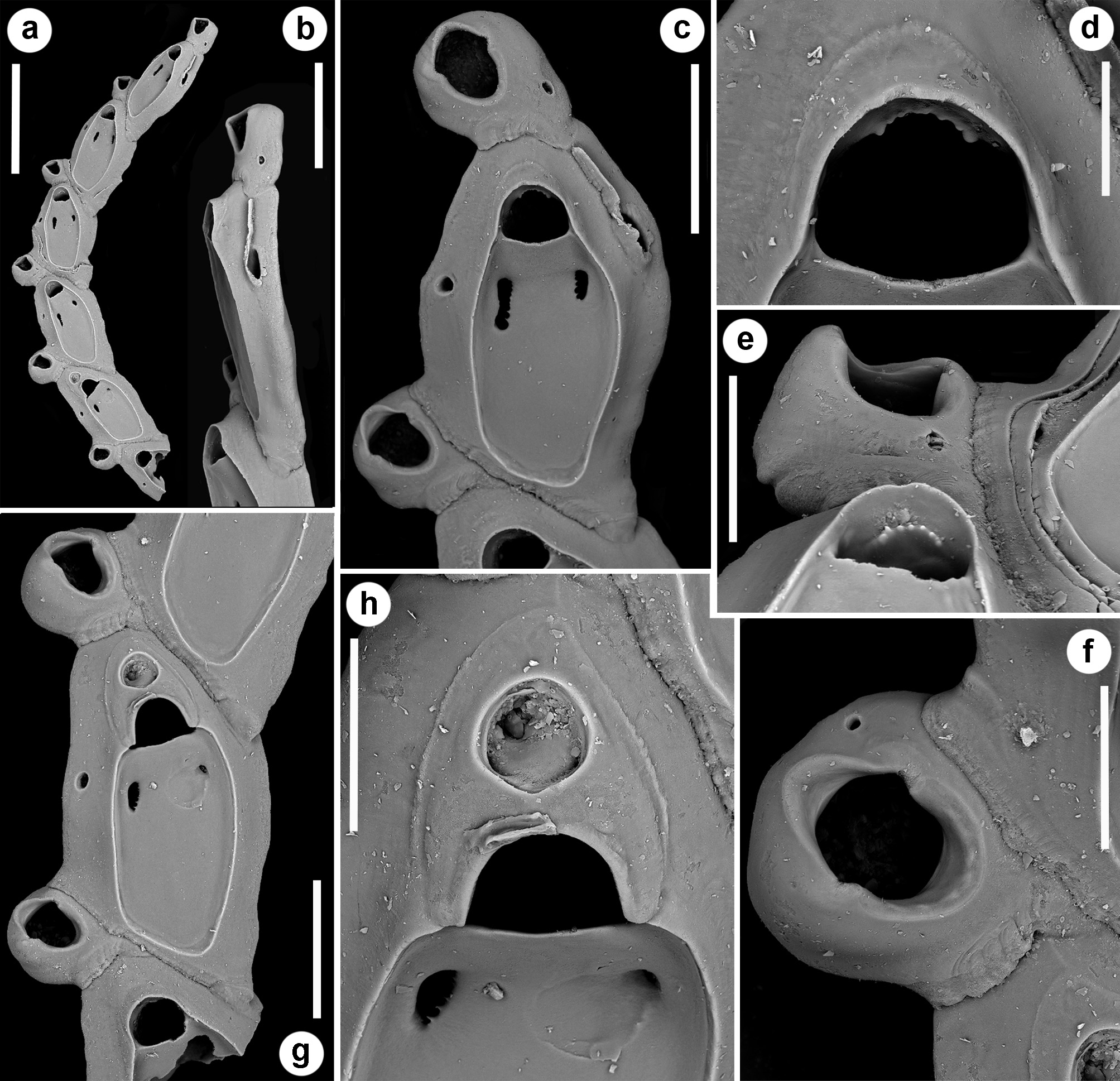
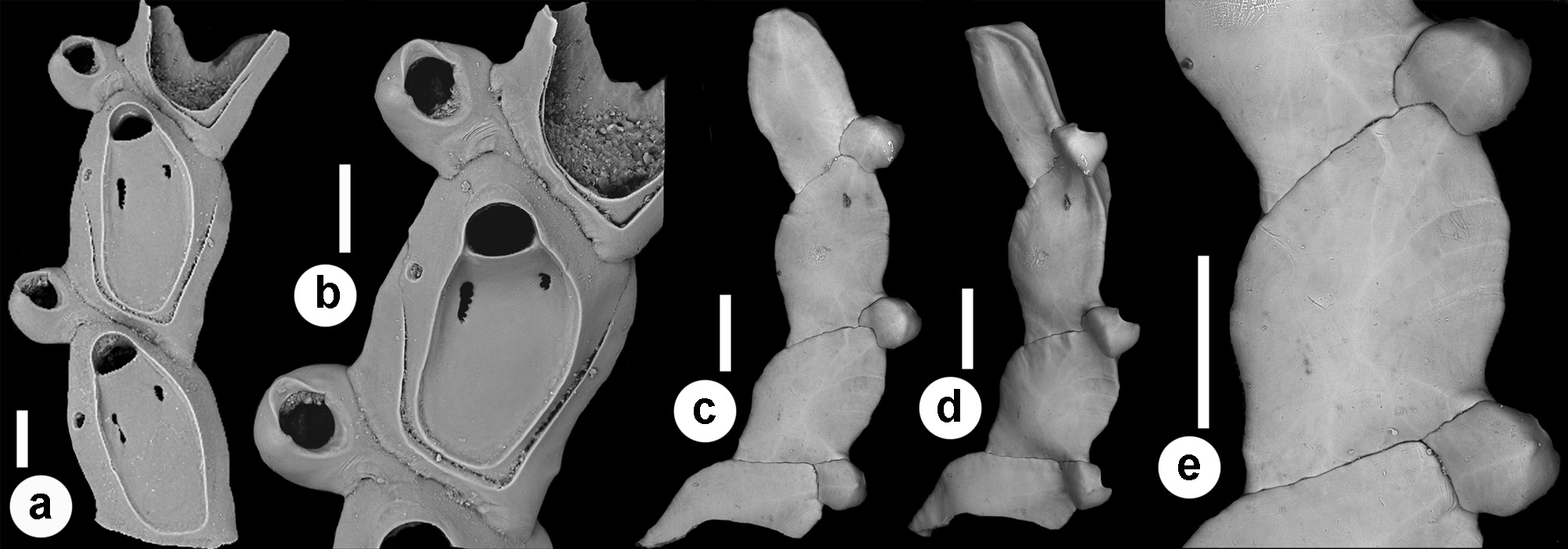
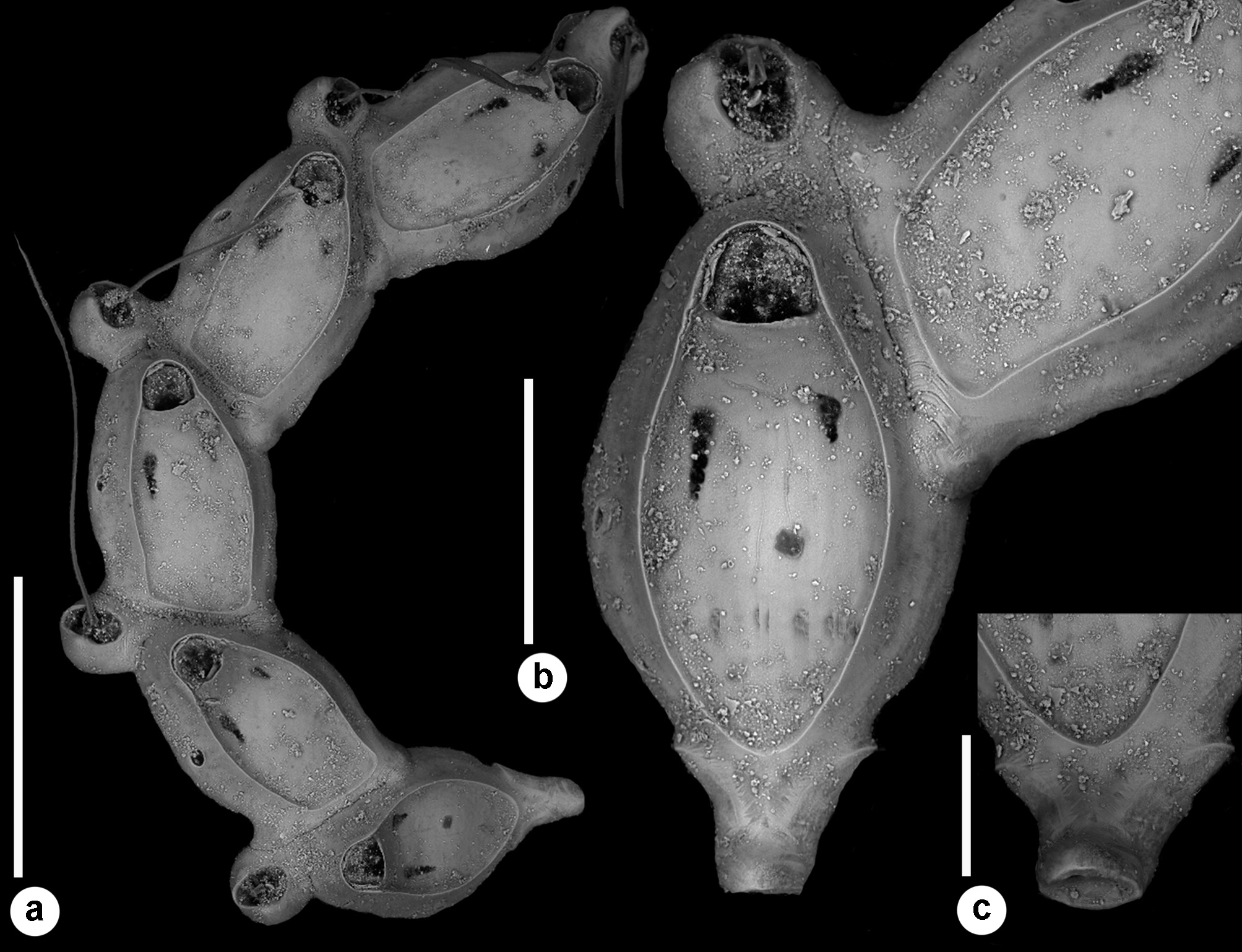
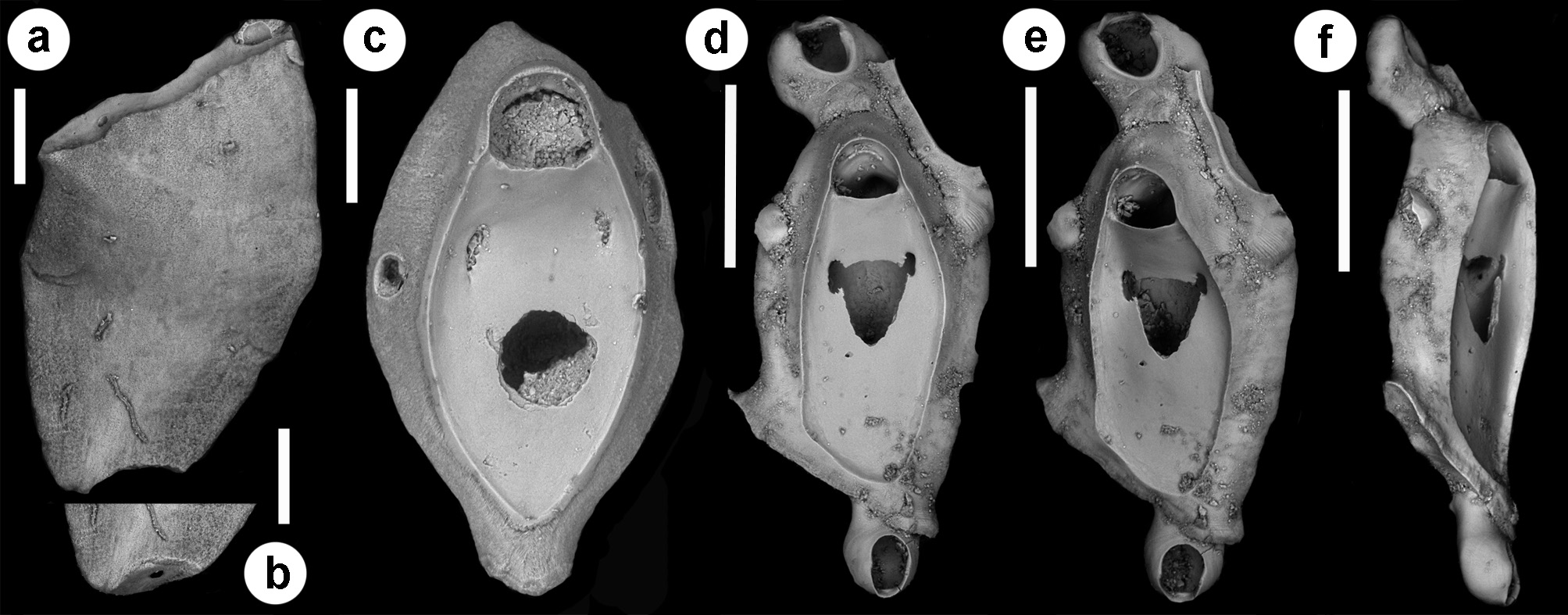
Description. Colony seemingly free-living, formed by a gently curved, scorpioid, uniserial row of zooids, budding in a clockwise, slightly elicoidal pattern ( Figs 18a View FIGURE 18 , 19c, d View FIGURE 19 , 20a View FIGURE 20 ). Each autozooid budding a distolateral daughter zooid from an elliptical septulum ( c. 50 µm long) located on the right side at an angle of c. 45° with its longitudinal axis, often in a slightly different plane; a nearly symmetrical, potentially active, circular septulum ( 15–30 µm in diameter) located on the left side. Autozooids thin in lateral view ( Fig. 18b View FIGURE 18 ), from c. 95 µm proximally up to c. 135 µm distally, elongate rhomboidal, asymmetrical, with an obliquely truncate proximal end and a nearly parallel distolateral right side, corresponding to the contact with the proximal and the distal zooids in the chain, respectively. Lateral gymnocyst extensively exposed and gently sloping towards the base ( Figs 18c, g View FIGURE 18 , 19a, b View FIGURE 19 , 21b View FIGURE 21 ). Frontal surface bordered by a raised, thin and smooth rim. Cryptocyst extensive, depressed, smooth and imperforate except for two opesiules, gently rising distally to form the proximal, slightly concave border of the opesia ( Figs 18c, g View FIGURE 18 , 19a, b View FIGURE 19 , 21b View FIGURE 21 ). Opesia transversely D-shaped, with rounded proximal corners and a deeply-located, arched, distal shelf, occupying one-third of the opesial width, and formed by a series of granulations, the two external the most prominent ( Fig. 18d, e View FIGURE 18 ). A pair of subcircular to elongate and irregularly-shaped opesiules placed in the most depressed area of the cryptocyst, c. 50 µm below the opesia; internal margin with a few, slender, pointed denticles, external margin smooth; asymmetrical, the left opesiule often being almost twice as long as the right one ( Figs 19a View FIGURE 19 , 20a, b View FIGURE 20 , 21a View FIGURE 21 ). Vibraculum subglobular, budded at the distal end of each autozooid, slightly inclined on the left side of the associated zooid and strongly protruding from the arched profile of the colony ( Figs 18a, c, g View FIGURE 18 , 19a, b View FIGURE 19 , 20a View FIGURE 20 ); cystid swollen with an acornshaped opesia and symmetrical blunt condyles separating the subelliptical talon with a slightly beaded border, from the bell-shaped, slightly flared rostrum with a short distal palatum ( Fig. 18e, f View FIGURE 18 ); mandibular seta slender, more than twice as long as an autozooid, directed outward ( Fig. 20a View FIGURE 20 ); circular ( c. 10–13 µm in diameter), uniporous septulum located on the right side, communicating with the distolateral autozooid. Terminal, subimmersed ovicell produced by a slightly shorter maternal autozooid, elongate, visible distal to the opesia as a gently swollen prominence defined by a faint, finely denticulate, flam-shaped line; ectooecium smooth with a relatively large window exposing the smooth endooecium with a median circular pore ( Figs 18g, h View FIGURE 18 ). Proximal border deeply arched and lateral wings almost reaching the opesial corners but leaving two lateral indentations producing dimorphic opesiae in ovicellate zooids ( Fig. 18g View FIGURE 18 ). Basal zooidal wall smooth and slightly convex, with barely visible radial lines; avicularium basal wall distally pointed ( Figs 19 View FIGURE 19 c–e, 21a, b). Ancestrula not observed. Two zooidal rows, one living and one dead, starting from symmetrical zooids tapering proximally in a tubular termination interpreted as subcolonies ( Figs 19c, d View FIGURE 19 , 20 View FIGURE 20 , see Remarks).
Remarks. Colonies of Setosella alfioi n. sp. are slender and fragile, as indicated by the fragmentary material available for study and the occurrence of several, broken and subsequently regenerated autozooids. The general morphology of the colony remains unknown, although the arrangement of the few zooids in the available fragments suggests that colonies are elongate scorpioid, slightly twisted and seemingly free-living or ring-shaped, like those of Setosella folini Jullien, 1882 . Like for S. folini (see Souto et al. 2011), the hypothesis that fragments could originate from detached and broken portions of other Setosella species (e.g. S. vulnerata , S. cyclopensis n. sp.) living in neighbouring habitats must be discarded. Both S. vulnerata and S. cyclopensis n. sp. encrust sand grains, usually not projecting beyond the substratum ( Fig. 4 View FIGURE 4 ) (see also Rosso 2008a, fig. 1). Furthermore, zooidal basal walls of these species typically are incompletely mineralised, leaving wide uncalcified windows and producing etching on the carbonate surface underneath ( Rosso 2008b, fig. 3I–J, L–M, O–P). The origin of the fragments from breakage of S. folini colonies is also unlikely. The latter species, recorded in the Atlantic Ocean ( Jullien 1882a; Harmelin 1977; Harmelin & d’Hondt 1992; Souto et al. 2011) and also off Marseille in the Mediterranean Sea ( Calvet 1907), is similar to S. alfioi n. sp. in having free-living colonies with a clockwise budding pattern, rhomboidal and thin autozooids, and in the position of vibracula. It differs in having autozooidal rows characterised by a narrower curvature, leading to the development of rings with a smaller diameter, often overgrowing one another, finely granular cryptocyst, zooidal opesia with marked proximal corners, vibraculum roughly co-axial with the proximal zooid and with a rounded opesia ( Souto et al. 2011). Furthermore, both autozooids and vibracula of S. folini are less elongate, being comparably shorter but wider ( Table 5 View TABLE 5 ).
A true ancestrula was not found. A few symmetrical, proximally tapering zooids with proximally located septula indicating potential connections with other zooids were interpreted as first zooids in ‘subcolonies’, presumably budded at the periphery of larger colonies. Although rare, peripheral budding has been documented for some discoporellid species ( Hakånsson & Thomsen 2001; O‘Dea et al. 2008; Bizzarri et al. 2015) and also suggested for S. folini , inferring the potential for this species to form on the left side of the colony similar symmetrical zooids tapering proximally, and easily detachable to become the first zooids of new colonies ( Souto et al. 2011, figs 21, 24, 27). An unizooidal specimen with the same morphology was also found isolated in the material from the Pleistocene of Archi ( Fig. 20c View FIGURE 20 ). An additional unizooidal fragment clearly shows evidence of a proximal vibraculum regenerated from the pore first used as connection with the proximal zooid in the chain ( Fig. 20 View FIGURE 20 d–f).
Intrazooidal budding is common: a single regeneration was observed in several zooids broken at about midlength, while two regenerations were observed in a zooid of the holotype. The common occurrence of intramural budding and regeneration of vibracula with reverse polarity, and the absence of a true ancestrula, might indicate fragmentation as an additional reproductive strategy.
Fragments comprising 3–8 zooids reported by d’Hondt (1981) as S. vulnerata and described as showing similarities with S. folini but with a larger curvature of the zooidal ring, may belong to S. alfioi n. sp. Examination of this material is needed to confirm the conspecificity.
Distribution. The only living colony fragment of Setosella alfioi n. sp. comes from cold-water coral habitats off Santa Maria di Leuca (NE Ionian Sea), collected at 513 and 790 m depth ( Mastrototaro et al. 2010). Dead specimens were collected in the SE Tyrrhenian Sea at the depth range 945–1521 m ( Di Geronimo et al. 2001). The possible occurrence of S. alfioi n. sp. in the Gulf of Sirte ( 33°57,0’ N, 15°08,2’E; 500–509 m) (d’Hondt 1981) needs to be confirmed after re-examination of the specimens. Isolated zooids of S. alfioi n. sp. found in the Pleistocene, bathyal sediment of Archi ( Calabria), date the species back to the MNN 19f Zone, corresponding to the late Calabrian or the Ionian ( Di Geronimo et al. 1997).
The presence of S. folini in the Mediterranean bryozoan fauna (see Rosso & Di Martino 2016) is still valid based on the record of Calvet (1907).
Ecology. Setosella alfioi n. sp. is exclusively recorded from deep waters ( 500–1521 m), associated with silty and muddy sediment, often containing coarser bioclastic fractions ( Di Geronimo et al. 2001; Mastrototaro et al. 2010). The inferred palaeoenvironment for the fossil associations, including the specimens studied here, is consistent with present-day observations ( Di Geronimo et al. 1997).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Flustrina |
|
Family |
|
|
Genus |
Setosella alfioi
| Rosso, A., Martino, E. Di & Gerovasileiou, V. 2020 |
Setosella folini :
| Di Geronimo 2001: 283 |
Setosella vulnerata :
| Di Geronimo 1997 |