Sarcoplana musculosa
|
publication ID |
https://doi.org/ 10.1093/zoolinnean/zlac072 |
|
publication LSID |
lsid:zoobank.org:pub:010109AB-79F5-4E6D-909B-08BB1803E589 |
|
DOI |
https://doi.org/10.5281/zenodo.7797680 |
|
persistent identifier |
https://treatment.plazi.org/id/03C49B73-6950-FF97-FCE0-753AB681718F |
|
treatment provided by |
Plazi |
|
scientific name |
Sarcoplana musculosa |
| status |
|
SARCOPLANA MUSCULOSA ALMEIDA & CARBAYO SP. NOV.
( FIGS 40–44 View Figure 40 View Figure 41 View Figure 42 View Figure 43 View Figure 44 )
Zoobank registration: urn: lsid: zoobank. org:act: 67BAB3EC-6FCC-4799-89E9-48133F0A40D1
Holotype: MNHNCL PLAT-15047 (Field code, F4886). Parque Nacional Nahuelbuta, Purén, Región de La Araucanía, Chile (37°49′′37.2′′S, 073°00′′32.4′′W), coll. F. Carbayo et al., 11 December 2010. Cephalic region: transverse sections on 11 slides; portion immediately behind the cephalic region: horizontal sections on six slides; pre-pharyngeal region: transverse sections on 17 slides; pharynx and copulatory apparatus: sagittal sections on four slides.
Type locality: Parque Nacional Nahuelbuta, Región de La Araucanía, Chile. The species only known from this locality.
Etymology: The specific epithet is from the Latin adjective musculosus, muscular, alluding to the thick cutaneous musculature.
Description
External aspect: At rest, the live specimen measured approximately 18 mm long and 3 mm wide ( Fig. 40A, B View Figure 40 ). The body length may double when crawling ( Fig. 40C View Figure 40 ). The body margins are parallel. The anterior tip is rounded, while the posterior is pointed. The dorsum is convex and the ventral side is flat. The preserved specimen measured 17.5 mm long, 2.5 mm wide and approximately 1.5 mm high.
The dorsum displays a pure orange (RAL 2004) median stripe 21% of the body width, which is divided longitudinally by a thin carmine-red (RAL 3002) midline (2.3% of the body width; Fig. 40A, C View Figure 40 ). The median stripe is absent in both extremities of the body. External to the median stripe is a black-red (RAL 3007) band 37% of the body width, the margins of which are darker. Some pure orange spots occur in the bands. These bands converge in the cephalic region. The ventromarginal sensory border is a line with beige-grey colour ( Fig. 40B View Figure 40 ). The body margins are pure orange. The ground colour of the ventral side is pure orange, provided with a pair of bands, each with 26% of body width, consisting of brown-red (RAL 3011) dots. The inner and outer margins of the bands are darker ( Fig. 40B View Figure 40 ).
The monolobate eyes measure 4 5–5 0 µm in diameter. They are distributed in an irregular row contouring the cephalic region and extending marginally to the posterior tip of the body. Sensory pits are absent. Instead, the ventromarginal epithelium of the cephalic region possesses sensory depressions. These depressions reach the underlying basal lamina and are provided with cilia ( Fig. 41A View Figure 41 ). The sensory depressions are absent at the anterior tip of the body. The mouth is positioned at a distance from the anterior extremity of the body equal to 66% of the body length; the gonopore 77%.
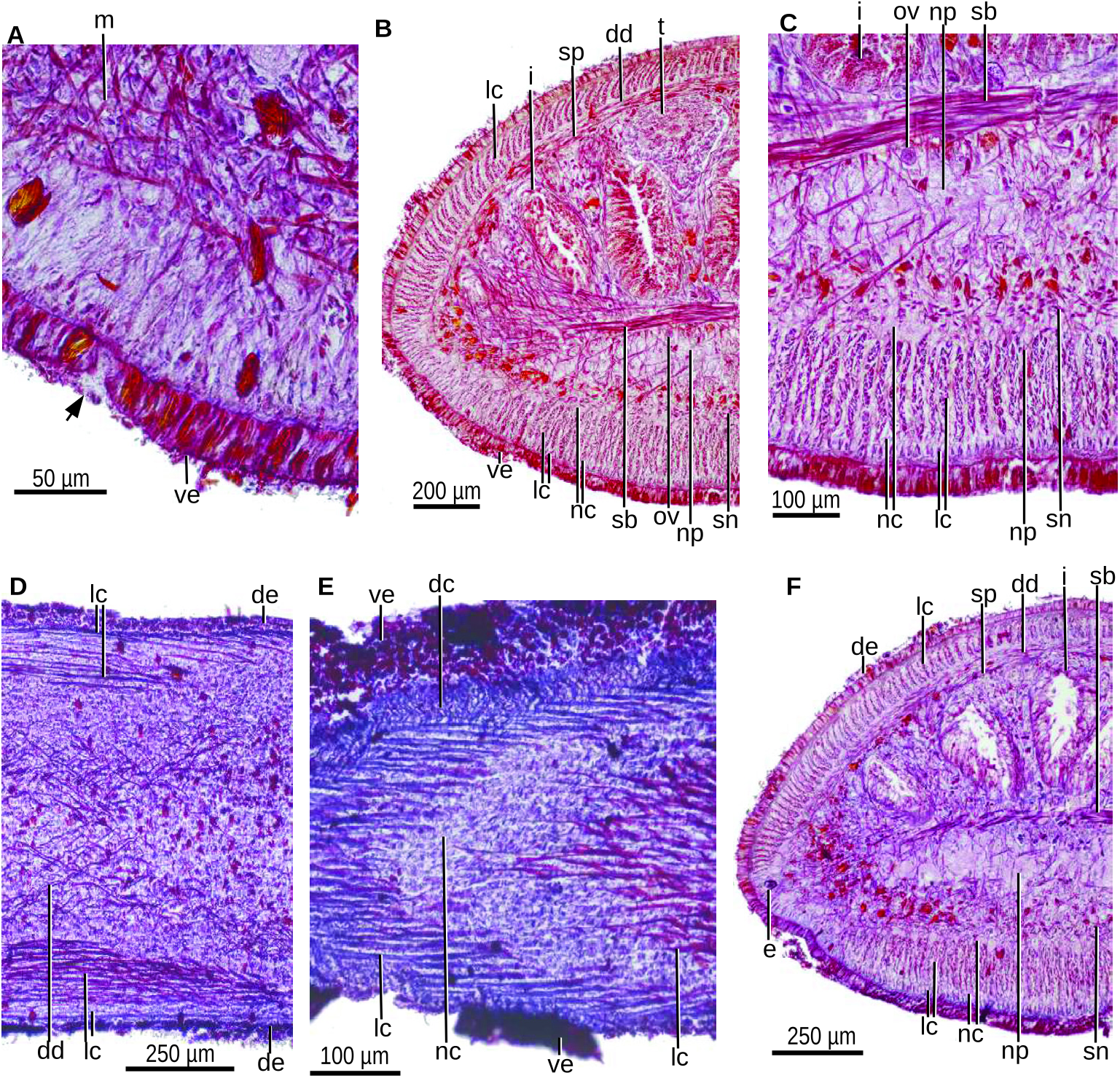
Internal morphology: The epidermis is ciliated only on the creeping sole, this occupying 83% of the body width. Gland cells producing erythrophil granules and cells producing rhabdites discharge through the entire epidermis. The erythrophil type is more abundant in the body margin, while the rhabditogen cells are more numerous in the ventral surface of the cephalic region ( Fig. 41A View Figure 41 ). Gland cells producing weakly cyanophil granules also discharge through the ventral and marginal epidermis. A glandular margin is absent.
The cutaneous musculature comprises three layers, namely, a subepidermal, layer (2 µm thick) of circular muscle, followed by a double layer (13 µm) with decussate fibres and an innermost layer of longitudinal muscle, the fibres of which are gathered into bundles ( Fig. 41B–F View Figure 41 ). This latter layer is 70 µm thick dorsally and 160 µm ventrally. It is thinner than the body margins, where it remains conspicuous ( Fig. 41B, F View Figure 41 ). The ventral layer of the longitudinal muscle is divided into a thin outer muscle and a thick inner muscle. These outer and inner ventral portions of the longitudinal muscle are separated by a secondary peripheral nerve net ( Fig. 41C View Figure 41 ). The relative thickness of the cutaneous musculature is 19.5% of body height.
The parenchymal musculature comprises four layers along the entire body: a dorsal layer (30 µm thick) of decussate fibres located to the inside of the peripheral nervous net; a dense layer of supraintestinal transverse muscle (40 µm); a dense layer of subintestinal transverse muscle (75 µm); and a subneural layer of decussate fibres (40 µm) ( Fig. 41B, C, E, F View Figure 41 ). Additionally, abundant oblique muscle fibres run in transverse body planes along the body.
The muscular organization changes in the anterior region of the body. At 1.9 mm from the anterior tip of the body, the longitudinal cutaneous muscle is 40 µm thick dorsally and 180 µm ventrally. In this region, the relative thickness of the cutaneous musculature is 21.6% of the body height ( Fig. 41F View Figure 41 ). The cutaneous and parenchymal muscles are thinner at 1.35 mm from the body tip. At 0.6 mm, the inner ventral cutaneous longitudinal muscle concentrates medially, so that one-quarter of the body width at each side of the body lacks this muscle. In this region, a cephalic retractor muscle is flat lenticular in cross-sections ( Fig. 42A View Figure 42 ). At 0.4 mm from the anterior tip, the secondary peripheral cutaneous nerve net is inconspicuous so that the ventral cutaneous muscle is no longer divided into an outer and an inner layer. Here, the longitudinal muscle is roughly lenticular in cross-section ( Fig. 42B View Figure 42 ). Toward the anterior tip of the body, the retractor muscle becomes progressively smaller as its muscle fibres progressively detach from it to run obliquely to the dorsum and body margins ( Fig. 42C, D View Figure 42 ).
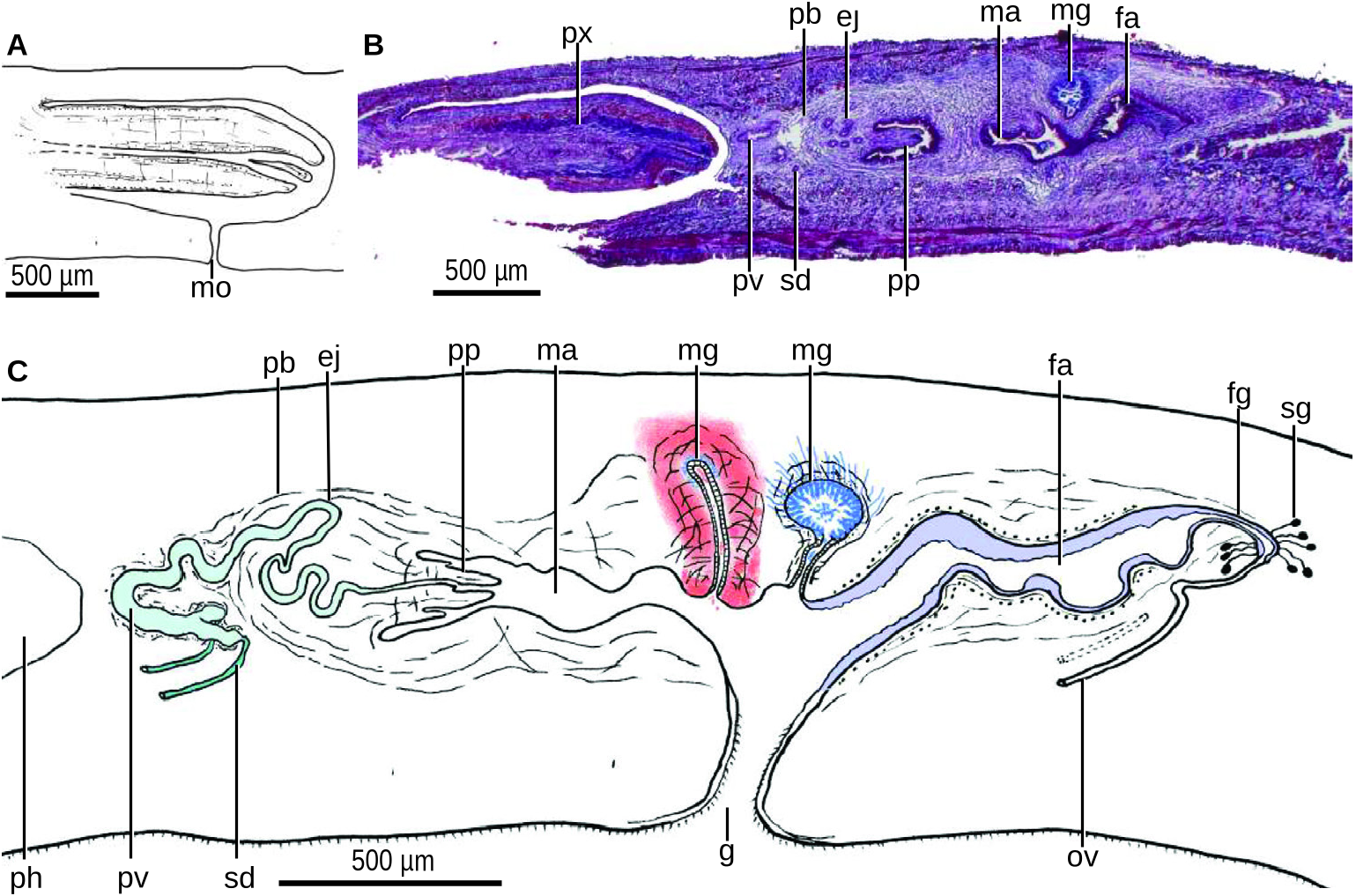
The mouth is located at a distance from the anterior region of the pharyngeal pouch, equivalent to 65% of the pouch length. The pharynx is cylindrical ( Fig. 43A, B View Figure 43 ). The ventro-anterior portion of the pharynx was cut off for DNA extraction, and thus the presence of an oesophagus could not be ascertained. The outer pharyngeal musculature consists of a subepithelial layer (5 µm thick) of longitudinal muscle, followed by a layer (8 µm) of circular fibres. The inner pharyngeal musculature consists of a single subepithelial layer of circular muscle, with longitudinal fibres interspersed (40 µm). The stroma of the pharynx has circular and longitudinal fibres.
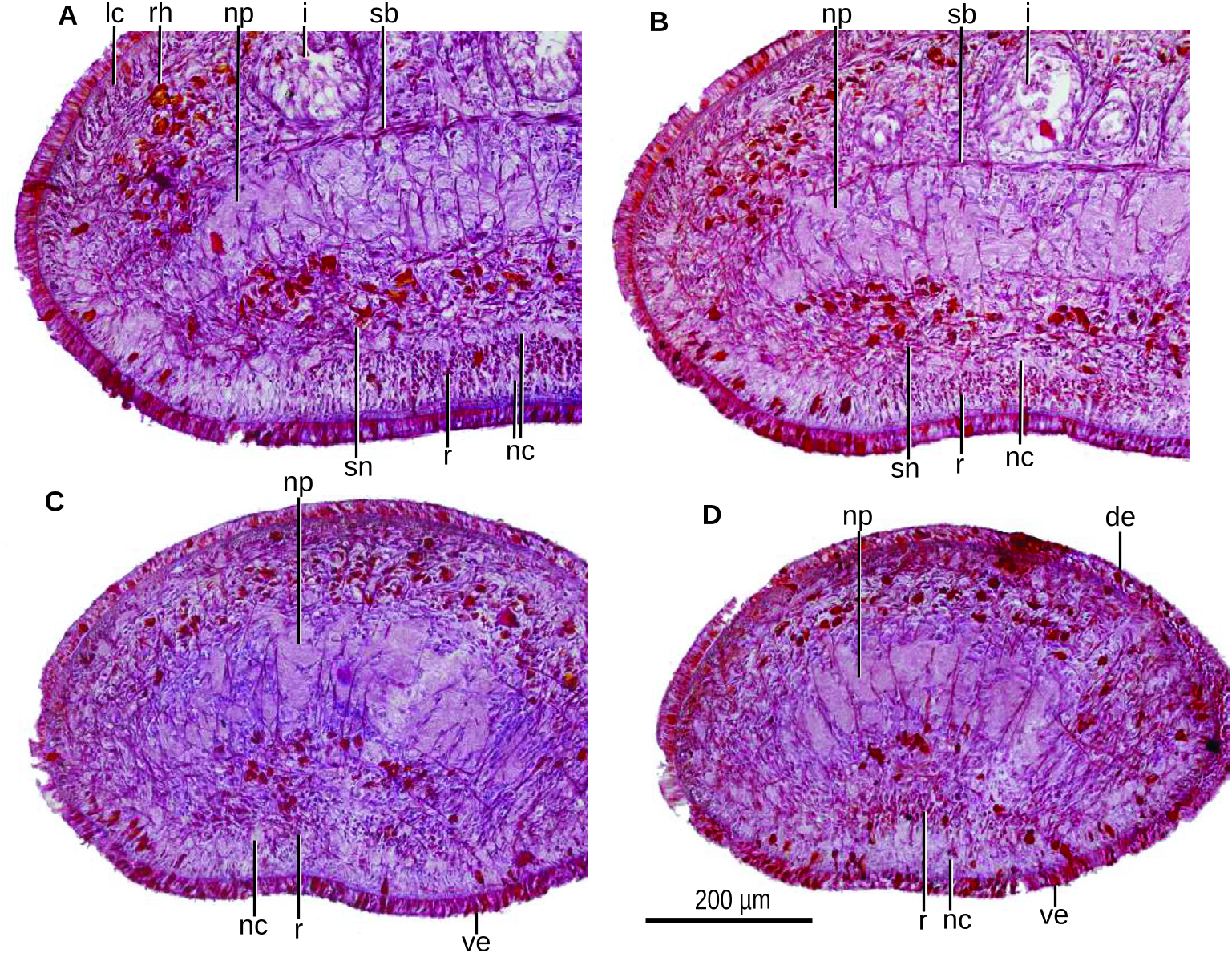
The testes are pear-shaped and measure approximately 400 µm high. They are dorsally located beneath the transverse supraintestinal parenchymal muscle and between the intestinal branches ( Fig. 41B View Figure 41 ). They are distributed in a row of one to two testes at each side of the body. The anteriormost testes are placed at a distance from the anterior tip of the body equivalent to approximately 35% of the body length, that is, 1.2 mm behind the ovaries; the posteriormost testes lie at a distance from the anterior tip equivalent to 44% of body length, that is, 100 µm anterior to the pharynx.
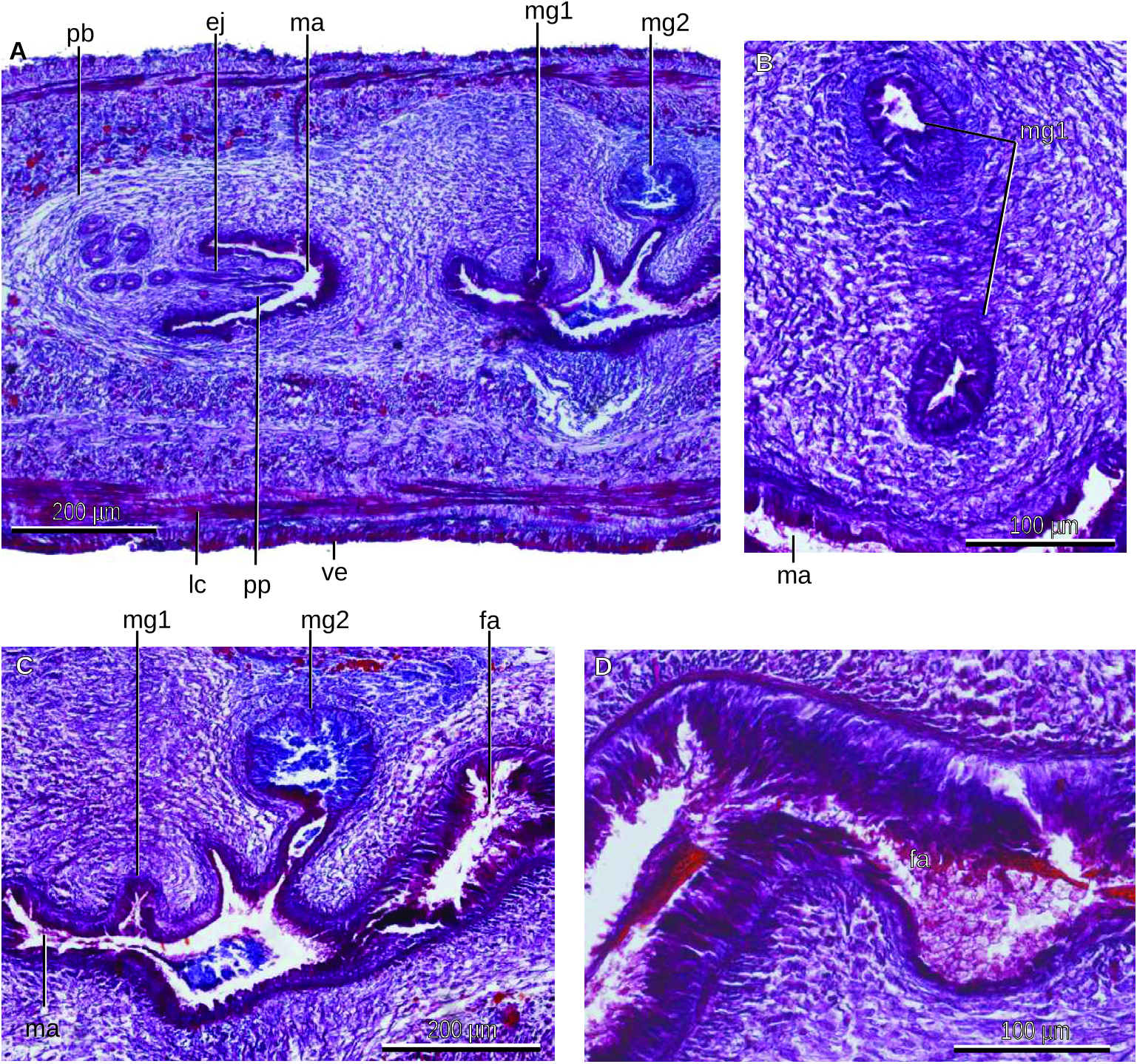
The sperm ducts run above the subintestinal parenchymal muscle and more or less dorsally to the ovovitelline ducts. The distal portion of the sperm ducts bends dorsally to the sagittal plane to open into the proximal section of the respective branch of the prostatic vesicle ( Fig. 43C View Figure 43 ). The prostatic vesicle is a sinuous tube roughly C-shaped in lateral view. Its proximal portion is bifurcate. This vesicle penetrates the anterior region of the penis bulb to join the ejaculatory duct. The penis bulb is well developed and is mainly constituted of longitudinal fibres. Most of the ejaculatory duct is sinuous and located within the penis bulb, while its distal section is straight and opens at the tip of the penis papilla. The penis papilla is 300 µm long and lies horizontally. This papilla is conical and presents some folds ( Figs 43C View Figure 43 , 44A View Figure 44 ).
The prostatic vesicle is lined with a cuboidal, apparently non-ciliated epithelium. This epithelium is pierced by the necks of gland cells producing fine (0.5 µm) erythrophil granules and is surrounded by a circular muscle (10 µm thick). The ejaculatory duct is lined with a cuboidal ciliated epithelium. The basal-half of the penis papilla is lined with a columnar epithelium traversed by the necks of numerous openings of gland cells producing erythrophil granules. The distal-half of the papilla is lined with a cuboidal epithelium through which some gland cells of the same type discharge. The epithelium of the penis papilla is underlain by some longitudinal muscle fibres.
The male atrium is narrow, elongated and roughly smooth ( Fig. 43C View Figure 43 ). This atrium is lined with a cuboidal-to-columnar epithelium, the apical surface of which is erythrophil. Numerous gland cells discharge erythrophil granules through the atrial epithelium, which is underlain by a layer (10 µm thick) of circular muscle, followed by a layer (10 µm) of longitudinal fibres. The atrial wall dorsal to the gonoduct presents the openings of two different musculoglandular organs, one located behind another ( Fig. 43C View Figure 43 ). The anterior organ (named mg 1 in the figures) consists of a 310 µm long and 30 µm wide, bowed and vertical blind duct embedded into the parenchyma and surrounded by muscle fibres. The duct of this musculoglandular organ is lined with a 10 µm high columnar epithelium, and the cells of this epithelium contain fine erythrophil granules (0.5 µm in diameter) produced by gland cells located outside the organ. The epithelium of the duct is underlain by a layer (10 µm thick) of circular muscle, followed by a layer (50 µm) of muscle fibres variously oriented, most circular. Beneath the epithelium of the innermost portion of the duct is a cyanophil, granular mass. The lumen of the canal contains some erythrophil granules.
The posterior musculoglandular organ (named mg 2 in the figures) is ampulla-shaped. It consists of a 130 µm long duct leading to a deeper, enlarged portion with 120 µm in diameter ( Figs 43B, C View Figure 43 , 44A View Figure 44 ). The duct is lined with a cuboidal, strongly erythrophil epithelium. A 30 µm thick longitudinal muscle underlies this epithelium. The cells of the lining epithelium of the enlarged portion are not discernible. Abundant gland cells with their bodies outside the organ discharge fine cyanophil granules into the lumen of the enlarged portion of the organ. Surrounding this enlarged portion of the musculoglandular organ is a 30 µm thick muscle net, followed by a layer (30 µm thick) of longitudinal fibres.
The ovaries are rounded-to-ovoid and approximately 100 µm in diameter. They are incompletely developed. These ovaries are located at a distance from the anterior tip of the body corresponding to 28% of the body length and 1.2 mm anterior to the anteriormost testes. The ovaries lie immediately above the ventral nerve plate.
The ovovitelline ducts emerge laterally from the dorsal side of the ovaries. Subsequently, these ducts run posteriorly above the nervous plate and immediately underneath the transverse subintestinal parenchymal muscle ( Fig. 41C View Figure 41 ). Just behind the level of the gonopore, one ovovitelline duct ascends gradually to enter the common ovovitelline duct behind the female atrium. This duct is short and oriented dorsally and communicates with the female genital canal. This female canal projects posteroventrally from the posterior wall of the female atrium ( Fig. 43C View Figure 43 ). The suboptimal quality of the sections did not allow examination of the second ovovitelline duct nor the type of epithelium lining the common ovovitelline duct and the female genital canal.
The female atrium is elongated and narrow. The dorsal wall of this atrium is more or less smooth, whereas the ventral wall is provided with three shallow recesses, each 100–200 µm in size ( Fig. 44C, D View Figure 44 ). The female atrium is lined with a columnar, 35–45 µm high epithelium. The free surface of this epithelium is erythrophil and resembles the bristles of a brush. Gland cells producing fine erythrophil granules pierce this epithelium. The recesses are lined with a low epithelium.
The female atrium contains clumps of xanthophil granules. The lining epithelium of the female atrium is surrounded by a 5 µm thick layer of longitudinal fibres, followed by a 10 µm thick layer of circular fibres. The male atrium to female atrium ratio is 84%. A common muscle coat wraps the distal-half of the prostatic vesicle and the male and female atria. This coat is comprised of abundant longitudinal muscle fibres.
Remarks on the neae tribe Sarcoplanini and its genera: The molecular phylogenies retrieved Sarcoplanini as a monophyletic group comprising Mapuplana , Pichidamas , Sarcoplana and Wallmapuplana . The intergeneric relationships are unstable. The monotypic genus Liana can be included in this tribe based on the morphological similarity of L. guasa Froehlich, 1978 with Sarcoplanini members, as shown below.
The species of Sarcoplanini share three unique characteristics among the Geoplaninae, namely, sensory depressions, a cephalic retractor muscle with a particular fibre organization (possibly secondarily lost in Wallmapuplana ) and a subneural parenchymal decussate muscle (but the fibre orientation of this muscle is unknown in Wallmapuplana ). These characteristics readily distinguish the Sarcoplanini members from the remaining Geoplaninae. Furthermore, branched glands associated with the prostatic vesicle are present in three of the four genera ( Mapuplana , Pichidamas and Wallmapuplana ).
Additional traits shared by all species in Sarcoplanini , and which probably evolved convergently in other lineages of Geoplanidae , are marginal distribution of the eyes [also present in Adinoplanini , Myoplanini , Haranini , Caenoplanini (Rhynchodeminae) and some Geoplanini)], a small penis papilla (e.g. Amaga , Gusana , but it is large in Liana ), a copulatory apparatus provided with musculoglandular organs [possibly secondarily lost in Mapuplana ; also present in Australasian taxa, such as some Bipalium , Artioposthia Graff, 1896 (see: Fyfe, 1947), Coleocephalus Fyfe, 1953 (see: Winsor, 1998)] and a female genital canal with the postflex condition (i.e. the canal approaching the female atrium from behind as in Pasipha , Gigantea , and Gusana , among others).
The genera of Sarcoplanini differ from each other by several structures. Sarcoplana stands apart from the remaining Sarcoplanini genera from the presence of a secondary peripheral nerve net located in the ventral side of the body (convergent in Myoplana ). Mapuplana and Liana are the only genera of Sarcoplanini having part of the ventral longitudinal cutaneous muscle sunken into the parenchyma. These two genera differ in that the penis papilla is small in Mapuplana (vs. large in Liana ). Wallmapuplana is the only genus of Sarcoplanini lacking a cephalic retractor muscle, while Pichidamas is the only genus having a large musculoglandular organ of adenodactyl type.
The genus Liana deserves a detailed discussion. This monotypic genus was proposed for L. guasa ( Froehlich, 1978) . The species was described from incompletely mature individuals. The main diagnostic features of the genus are: elongated body, broad creeping sole, sensory depressions (‘minute sensory pits’ in the original description), longitudinal ventral cutaneous muscle partially sunken into the parenchyma, cutaneous muscle thickness relative to the body height is 10%, testes are dorsal; copulatory apparatus without adenodactyls; penis papilla short and blunt; female canal approaches from horizontal or ventral aspect ( Froehlich, 1978). This species also has a subneural layer of decussate parenchymal muscle (‘a layer of fibres obliquely oriented to the right and to the left’ in Froehlich, 1978: 21) interwoven with fibres of the sunken longitudinal cutaneous muscle. The relative thickness of the cutaneous musculature increases to 21% when the sunken muscle portion is also considered (see: Froehlich, 1978: fig. 24).
There are no gene sequences available of this species. Among the Geoplanini tribes, Liana fits well Sarcoplanini :the creeping sole is wide; the eyes marginal; sensory depressions and subneural parenchymal decussate muscle are present. The original description of L. guasa does not mention a cephalic retractor muscle but a modification of the musculature organization in the cephalic region, which is compatible with a retractor organ (‘ At the anterior end, the dorsal longitudinal [Ʋentrally?] cutaneous fibers bend dorsally to end on the basement membrane. Laterally, toaeards the Ʋentral sensory border the cutaneous musculature progressiƲely loses height becoming minimal if not absent. Ventrally it regains height toaeards the median line, attaining a little more than half the height of the dorsal portion. […] The Ʋentral longitudinal parenchymal [cutaneous?] musculature progressiƲely disappears toaeards the anterior extremity. At the same time it appears there a layer of diagonal fibers interspersed aeith rarer and rarer longitudinal fibers. Presumably the longitudinal fibers change direction anteriorly but it cannot be discerned’, E. M. Froehlich, 1978, p. 22). The presence of two taxonomically relevant diagnostic features of Sarcoplanini , namely, branched glands associated with the prostatic vesicle and genital musculoglandular organs, could not be verified since the individuals are only partially mature.
Liana does not fit in any of the remaining tribes because it lacks the following features: carinate dorsal side, musculoglandular organs in the female atrium ( Adinoplanini ); body leaf-like with dorsal eyes (Geoplanini); anterior region of the body triangular, ventral and dorsal longitudinal cutaneous muscle sunken into the parenchyma, sensory pits ( Gusanini ); long pharyngeal pouch ( Haranini and Timymini); dilated female genital ducts ( Inakayaliini ); transneural parenchymal muscle of diagonal fibres ( Myoplanini ); extraordinarily wide and flattened body, marginal eyes, subneural parenchymal of transverse muscle and a transneural parenchymal layer of longitudinal muscle ( Polycladini ); semi-lunate head plate (Timymini). Therefore, we place the genus Liana in Sarcoplanini .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Order |
|
|
Family |
|
|
Genus |