Pyrgopsella youngi, Achituv, Yair & Simon-Blecher, Noa, 2006
|
publication ID |
https://doi.org/ 10.5281/zenodo.273558 |
|
DOI |
https://doi.org/10.5281/zenodo.5687280 |
|
persistent identifier |
https://treatment.plazi.org/id/038887B5-FFD7-2248-405C-0912FBB6FC64 |
|
treatment provided by |
Plazi |
|
scientific name |
Pyrgopsella youngi |
| status |
sp. nov. |
Pyrgopsella youngi sp. nov. Achituv
( Figs 1–6 View FIGURE 1. A View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 )
Material examined: Fifteen specimens, six with soft parts, were obtained from a partly bleached colony of Symphyllia radians MilneEdwards and Haime , found in a tropical fish shop in Israel. The source of the coral was Sulawesi, Indonesia, exact location unknown. The barnacles were suspended in the coral tissue and easily detached from the coral. Part of the host coral (catalogue number RMNH Coel. 33023) and the barnacle holotype ( RMNH C. 2602: two slides with cirri and mouth parts, SEM stubs of shell and opercular valves) and two paratypes ( RMNH C 2603 and RMNH C 2604: shell and opercular valves, and SEM stubs with shell and opercular valves, respectively) have been deposited in the RMNH. The other part of the host coral (catalogue number TAU Co32349), the remaining paratypes, shell and opercular valves of four specimens prepared for SEM analysis, slides of two specimens with cirri and mouth parts, are deposited in the TAU (catalogue number TAU Ar27804). Two specimens were used for DNA extraction.
Etymology: The specific epithet honors the late Paulo Young in recognition of his contribution to our knowledge of cirripede biology.
Diagnosis: Wall concrescent, ribs on shell absent. Basis, membranous, with inferior end calcareous. Scutum and tergum not fused, scutum transversally elongated, tergum triangular with internally directed tooth.
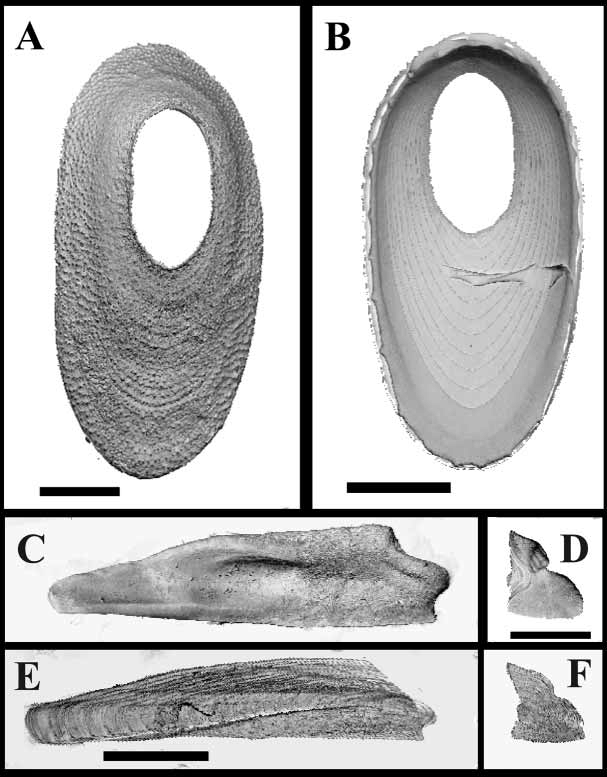
Description: Shell ( Fig. 1 View FIGURE 1. A C, 2A, 3A, B) white but nearly transparent, concrescent, lowconical, thin, oval, maximum carinorostral diameter 8 mm, maximum lateral diameter 4 mm. Radiating rib absent; shallow, concentric growth lines on outer surface. Shell tubiferous, tubes wide, penetrating 2/3 of shell; inner shell white; sheath with concentric growth lines, reaching margins of shell; short spines on sheath perimeter located at margins of lateral septa, number of spines equal to number of septa, with no denticulation on margins of septa. Orifice oval, located at carinal end of shell; carinorostral diameter 1/3 carinorostral diameter. Tergoscutal flaps banded purple on white cream ground. Basis ( Fig. 1 View FIGURE 1. A B) membranous, sometimes with basal part elongated and pedunclelike, basal tip connected to small, white, calcareous disc, latter usually attached to septum of coral calyx ( Fig. 3 View FIGURE 3 A,B).
Scutum and tergum white, separate. Scutum ( Fig. 2 View FIGURE 2 C, E) transversally elongated, thin, total length (including tergal tooth) ~five times maximal width; basal margins slightly sinusoidal; adductor muscle pit shallow, distinct; adductor ridge small, low; lateral depressor muscle pit indistinct; width of tergal tooth ~1/2 width tergal margin, located closer to occuludent margin then to basal margin; growth lines on outer surface; narrow, oblique furrow beginning at tergal margin halfway between tergal tooth and occludent margin, ending near occludent basal angle. Tergum ( Fig. 2 View FIGURE 2 D, F) triangular, growth lines on outer surface; short, spur barely distinct; external groove running from middle of scutal margin to basicarinal apex; small notch in middle of scutal margin; basiscutal angle pointed; thin, pointed, inwardprojecting tooth on spur; notch in middle of carinal margin; inner side with depression accommoding tergal tooth of scutum; growth lines visible inside depression.
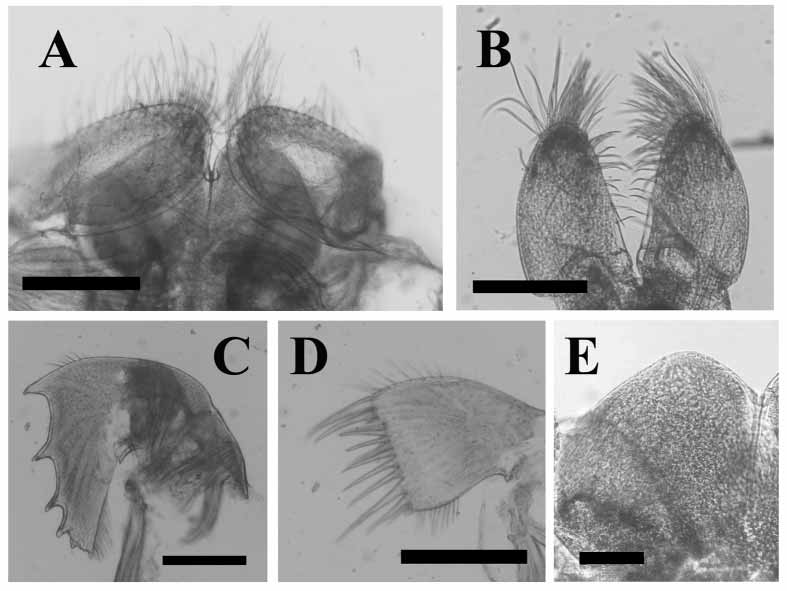
Labrum ( Fig. 4 View FIGURE 4 A, E) rounded with deep median notch, very short, fine setae on margins and outer surface; palps ( Fig. 4 View FIGURE 4 A) elongate, oval with upper margins straight, long setae on outer surface, longest at distal end, inner margins with shorter setae. Mandible ( Fig. 4 View FIGURE 4 C) with five teeth along cutting edge, distances between teeth unequal, upper two teeth occupying 1/2 length cutting edge, second and fourth teeth bifid, lower angle with short spines; face and margins of mandible bearing setae, lower margins with combs, conical spines on lower angle. Maxillule ( Fig. 4 View FIGURE 4 D) with cutting edge unnotched, armed with row of ~ten unequal spines, tuft of smaller, hairlike setae at distal angle; setae scattered along upper part of maxillule, row of dense setae on lower part; some combs of hairs on face of maxillule projecting beyond cutting edge. Maxilla ( Fig. 4 View FIGURE 4 B) ovalelongate, long setae distally, short setae on inner side, few simple setae on upper and lower margins, distal surface with two rows of short, sharp setae; lower angle with two to three spines.
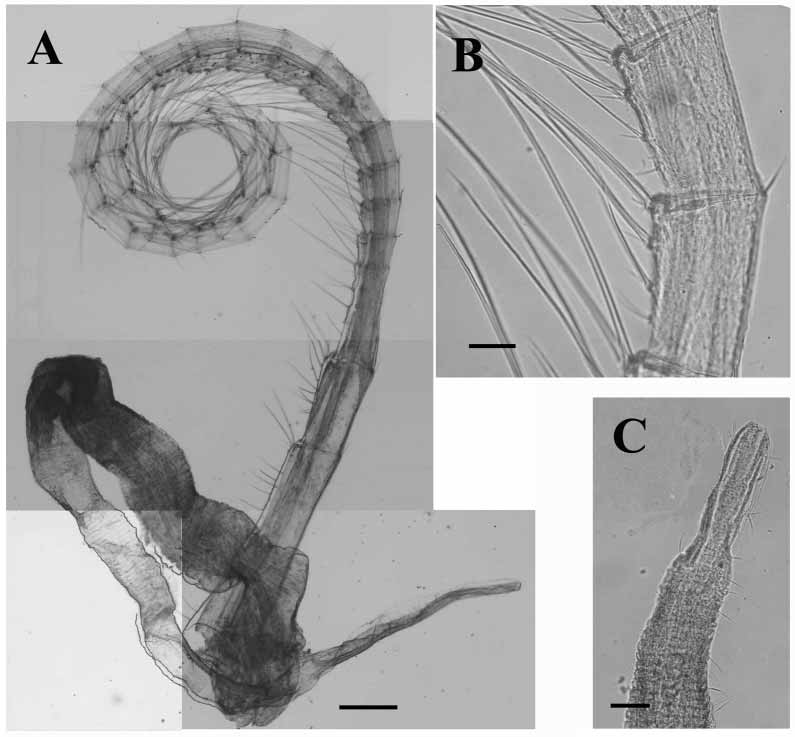
Number of articles of cirri of three randomly selected individuals given in Table 1 View TABLE 1 . Cirrus I ( Fig. 5 View FIGURE 5 A) highly setose; anterior ramus ~twice length of posterior ramus; proximal articles of anterior ramus and all segments of posterior ramus with protuberances bearing setae. Cirrus II ( Fig. 5 View FIGURE 5 B) with rami ~equal length; articles slightly protuberant, bearing long setae. Cirrus III with anterior ramus somewhat longer than posterior ramus; articles with protuberances bearing tufts of long, plumose setae ( Fig. 5 View FIGURE 5 C). Cirri IV ( Fig. 5 View FIGURE 5 D) to VI ( Fig. 6 View FIGURE 6 A) long and slender; each article with four pairs of setae of different lengths arranged along anterior margin, generally each pair accompanied by short, proximal seta; setae at distal end of articles longer, ~three times article width; two or three short setae at posterior articulation of each article ( Fig. 6 View FIGURE 6 B); basal articles with a row of pairs of setae.
Penis ( Fig. 6 View FIGURE 6 A) long, annulated, scattered thin setae along length; distal end modified, more slender then rest of penis, not annulated, few setae ( Fig. 6 View FIGURE 6 C).
Remarks: The barnacles from Symphyllia radians differ from those described by Gruvel (1907) in two striking aspects: the tergum as depicted and described by Gruvel carries a truncate internal tooth, whereas the inward projecting tooth found in our specimens is thin and pointed and located on the short spur. The second difference concerns the distal end of the penis. In Pyrgopsella annandalei it is ornamented with short spines, which are missing in our material, and the modified end of the penis in Gruvel’s material is pearlike, while in the present material this part of the penis is elongate. On the basis of these differences, we conclude that our material is not referable to P. annandalei , but should be assigned to a new species, named by us as P. youngi .
In most coralbarnacles the shell is overgrown by the coral and calcareous material is deposited on the barnacle surface. In many cases the coral forms spines or perturbations over the surface of the shell plates and in some cases even an entire corallite. However, in Pyrgopsella , soft coral tissue covers the shell with no deposition of calcareous material over it and the barnacle comes to be suspended in the coral tissue. A more remarkable difference between Pyrgopsella and other pyrgomatids is the membranous basis with a vestigial, calcareous part. In all other coralinhabiting barnacles the basis is calcareous and, in most cases, it is conelike and tapered, embedded in the coral skeleton. A particular feature of the coral Symphyllia radians is the thick, fleshy tissue in which the barnacles are embedded. This thick tissue supports the barnacles, thus making a calcareous basis superfluous.
Rosell (1973; 1975) reported the existence of a barnacle similar to Pyrgopsella annandalei embedded in a sponge collected in the Philippines. He described this barnacle as a new species, P. stellula . Grygier (1992) reported the occurrence of P. stellula in Japan, extending the geographical distribution of this species, and noted an additional species of Pyrgopsella in a sponge from Indonesia. Rosell (1973; 1975) suggested that the genus Pyrgopsella consists of sponge inhabiting barnacles and assumed that the host of P. annandalei was also a sponge. This view has been widely accepted, but our new find suggests that Pyrgopsella is a coralbarnacle and that Gruvel’s species, which is similar to ours, most probably was detached somehow from a coral.
It is surprising that for a century, ever since its description by Gruvel (1907), Pyrgopsella was never reported from corals. Both P. annandalei and P. youngi are rather large pyrgomatids, reaching a carinorostral diameter of 8 mm, and can hardly be missed. The lack of records until now can be explained by the way in which coralinhabiting barnacles are studied. Mostly, the barnacles are extracted from dry corals kept in museum collections. Moreover, since identification of corals is based on the structure of the calices and septa, in many cases biologists remove the coral tissue using household bleach or an alkali solution, whereby Pyrgopsella with its membranous basis would be lost. Examination of the dried and bleached skeleton of the coral from which the present sample was removed showed no conspicuous evidence of the presence of the barnacles. Only detection of the vestigial calcareous basis could show that barnacles had formerly lived on the coral. In the collection of Naturalis, Leiden, there is a colony of Symphyllia radiance from S.W. Sulawesi (RMNH Coel 24745) with vestigial calcareous basis. Our new findings underline the importance of examining live corals, or corals preserved with their tissue, for the presence of barnacles, a technique that might reveal additional kinds of coralinhabiting barnacle.
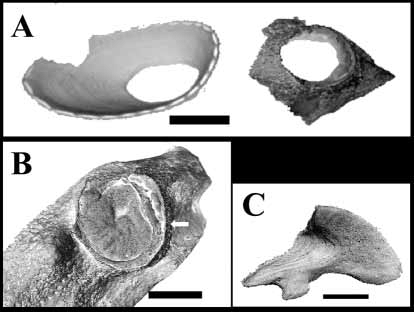
The main feature that Pyrgopsella stellula ( Figs. 1 View FIGURE 1. A C; 7A, B), P. annandalei (Pl. 2 Fig. 7 View FIGURE 7 b in Gruvel, 1907) and P. youngi ( Fig. 2 View FIGURE 2 A, B) share is a singleplate shell, but otherwise it is difficult to accept that these species all belong to the same genus. In addition to being symbionts of hosts from different phyla, there are striking morphological differences between these species. Pyrgopsella stellula lacks the peduncle that is present in P. annandalei (Pl. 2 Fig. 7 View FIGURE 7 a in Gruvel, 1907) and in P. youngi ( Fig. 1 View FIGURE 1. A B, D). In our material, and one may perhaps assume also in P. annandalei , the basal tip of the peduncle anchors the barnacle to the septa of a coral calyx. The shell of P. stellula is thin and flexible and only partly calcified ( Fig. 7 View FIGURE 7 A, B) The calcareous area is in the form of thin spokes radiating from the operculum and a flexible membrane connects those spokes. In P. youngi and P. annandalei , the shell is totally calcified. In P. stellula there is no distinct sheath, whereas it is distinct in P. annandalei and P. youngi ( Fig. 2 View FIGURE 2 B). The sickleshaped, barbed bristles and starlike spines ( Fig. 7 View FIGURE 7 G) that are arranged in concentric rows over the outer shell membrane in P. stellula are absent in P. youngi . The shape of the opercular valves of P. stellula ( Fig. 7 View FIGURE 7 C, D, E, F) and those of P. annandalei (Pl. 2 Figs 9,10 in Gruvel, 1907) and P. youngi ( Fig. 2 View FIGURE 2 C, D, E, F) are different. The scutum of P. stellula is elongated, rather big and lacks a tergal tooth ( Fig. 7 View FIGURE 7 D); in situ it projects beyond the rostral margins of the shell ( Fig. 1 View FIGURE 1. A D; see also Rosell 1975: Fig. 2 View FIGURE 2 c). The tergum is missing the internal tooth that is found in the other species of Pyrgopsella ( Figs 2 View FIGURE 2 D, 7C). Cirrus III of P. stellula carries small conical spines that are not found in P. youngi and cirrus IV of P. stellula is armed with diagonally arranged conical spines ( Rosell 1975: Fig. 2 View FIGURE 2 n) that are absent in P. youngi . On the basis of these differences we suggest that P. youngi sp. nov. and P. annandalei do not share the same evolutionary line as P. stellula and that the latter should be assigned to a different genus. Therefore, we propose a new genus, Pyrgospongia , to accommodate the spongeinhabiting pyrgomatid Pyrgospongia stellula ( Rosell 1973) .
TABLE 1. Cirral counts of three randomly selected specimens of Pyrgopsella youngi from Symphyllia radians, expressed as a – b for numbers of articles in anterior (a) and posterior (p) rami, respectively, of each cirrus; + indicates a broken ramus, R = right cirrus, L = left cirrus.
| Cirrus | I | II | III | IV V | VI | |
|---|---|---|---|---|---|---|
| Specimen 1 | R | 15–8 | 10–8 | 12–9 | 30–15+ 29–26 | 29–24+ |
| L | 15–7 | 9–8 | 12–9 | 32–23 28–23 | 28–28 | |
| Specimen 2 | R | 13–8 | 8–7 | 10–8 | 20–18 18–+ | 5+23 |
| L | 16–7 | 9–7 | 10–8 | 23–20 +26 | 20–24 | |
| Specimen 3 | R | 16–8 | 8–8 | 12–12 | 23–4+ +22+ | 30–26 |
| L | 17–7 | 7–7 | 10–9 | 26–22 23–24 | 13+25 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |