Protimesius lucifer, Osvaldo Villarreal & Ludson Neves de Ázara & Kury, 2019
|
publication ID |
https://doi.org/10.1080/00222933.2019.1620893 |
|
DOI |
https://doi.org/10.5281/zenodo.3679647 |
|
persistent identifier |
https://treatment.plazi.org/id/921387C6-126C-FFEF-DD93-FAA8FE1EFB15 |
|
treatment provided by |
Valdenar |
|
scientific name |
Protimesius lucifer |
| status |
sp. nov. |
Protimesius lucifer View in CoL sp. nov.
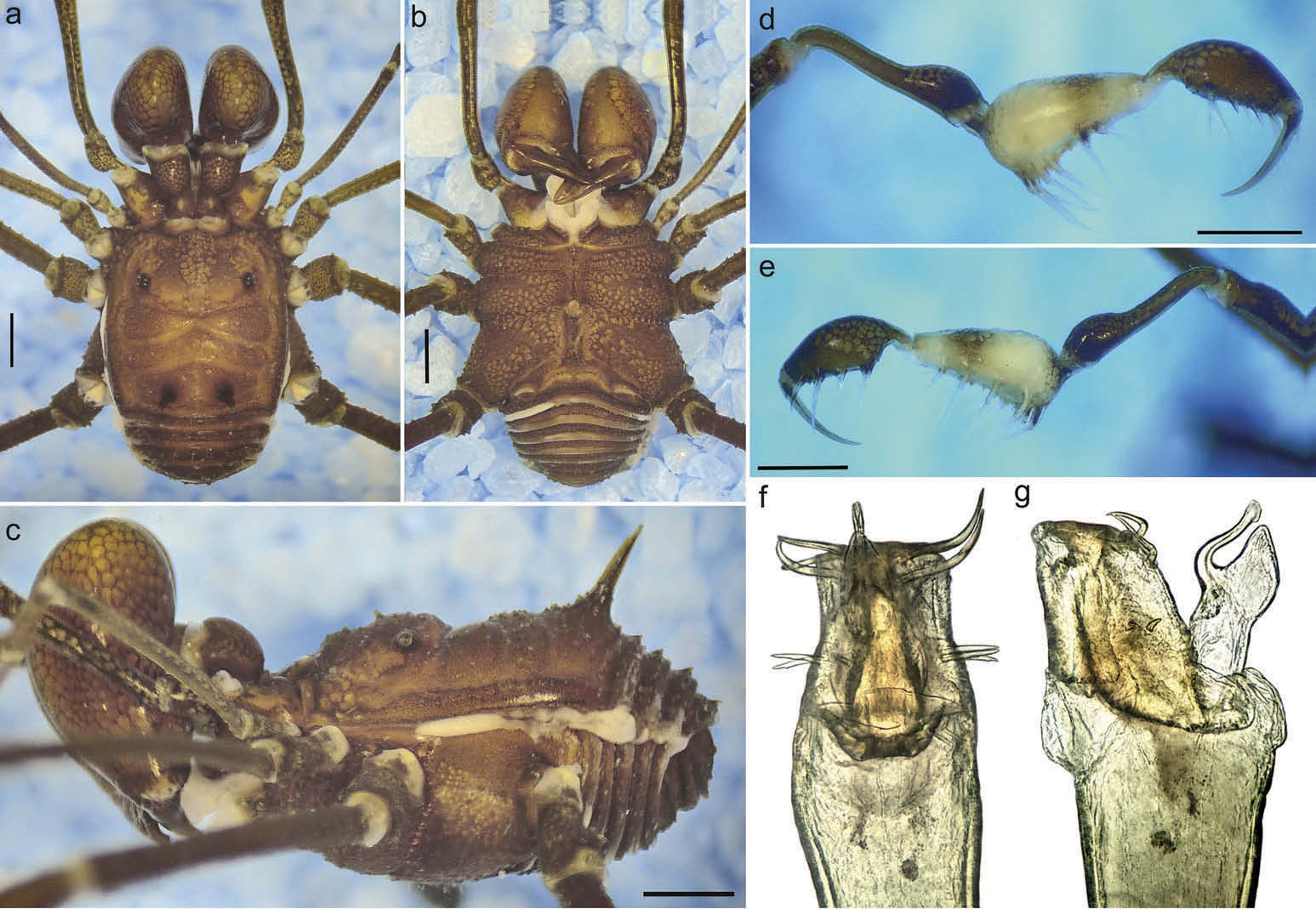
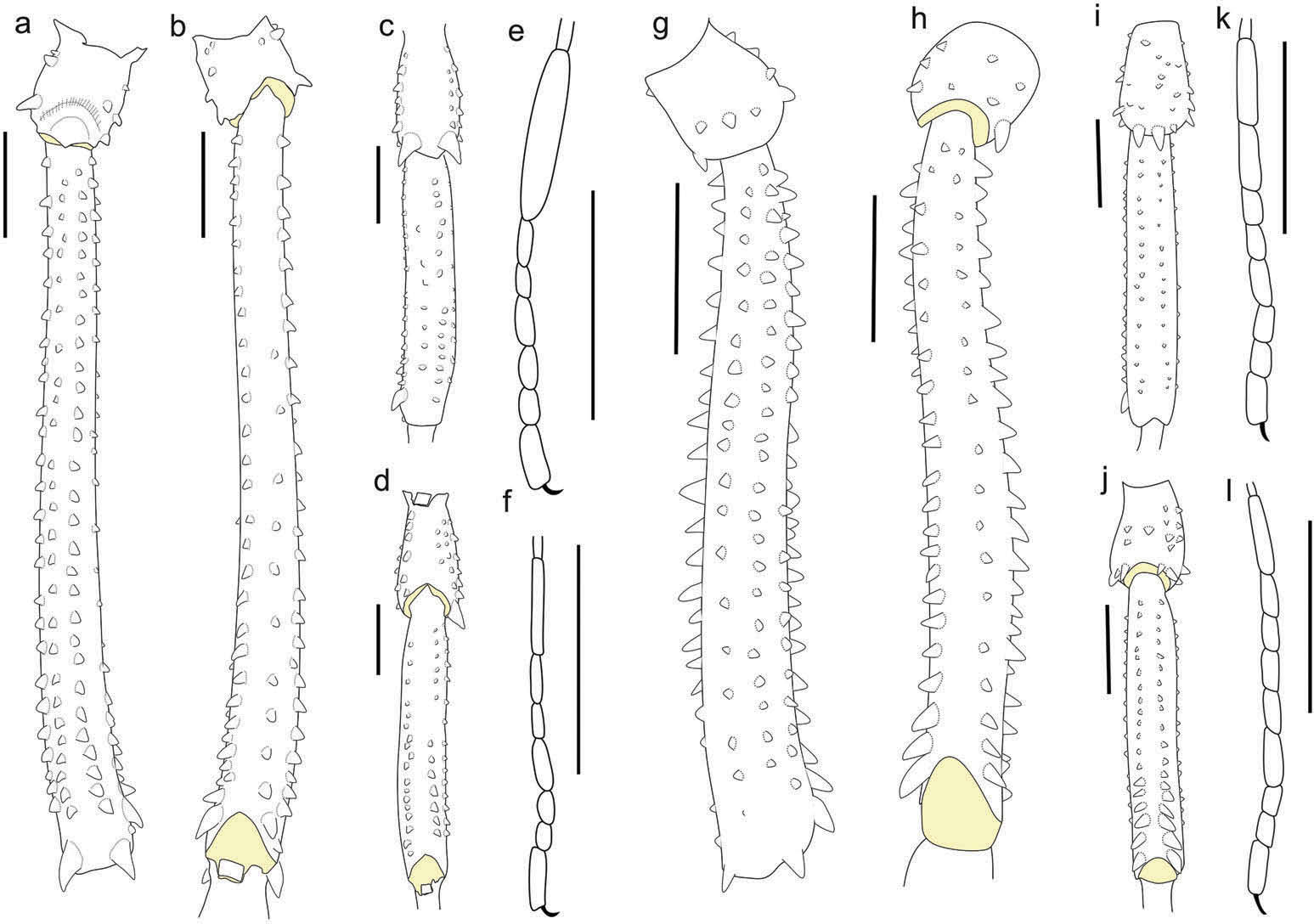
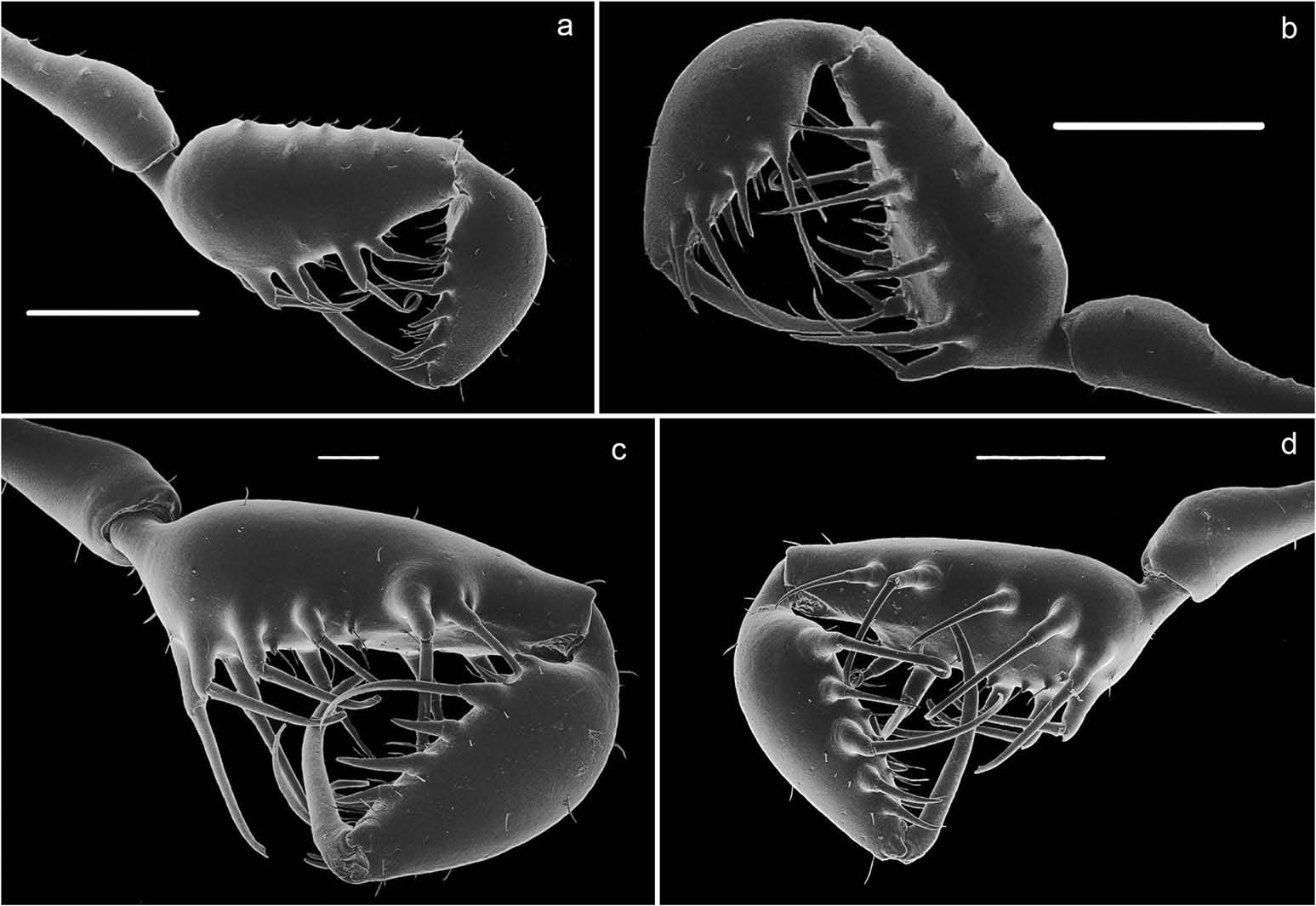
( Figure 1 View Figure 1 , 3 (a,c), 4 (a – d), 5 (a,b), 7 (a – c), 8 (a – d), 10)
Type data
³ holotype ( MNRJ 2560 ), BRAZIL, Pará, Parauapebas , Caverna GEM-1183 [−6.107330°, −50.133909°], 29.viii. 2010, Espeleo Team leg . Paratypes: 2 ³ ( MNRJ 9252 View Materials ), BRAZIL, Pará, Canaã dos Carajás, Caverna S11C-181 [−6.408810°, −50.395838°], 28.viii. 2015, Espeleo Team leg. 4 ³ ( MNRJ 2559 View Materials ), BRAZIL, Pará, Curionópolis, Caverna SL-72 [−5.972711°, −49.624495°], 23.vii. 2010, Espeleo Team leg.; 2 ³ ( MNRJ 19404 View Materials ), BRAZIL, Pará, Parauapebas, Caverna GEM-1183 [−6.107330°, −50.133909°], 29.viii. 2010, Espeleo Team leg; 1 ³ ( MZSP 64895 View Materials ), BRAZIL, Pará, Parauapebas, Caverna S11D-074 [−6.392278°, −50.318503°], 13 – 30.i. 2010, R. Andrade et al. leg.; 1 ³ ( MZSP 64893 View Materials ), BRAZIL, Pará, Parauapebas, Caverna S11D-019 [−6.403000°, −50.370789°], 13 – 30.i. 2010, R. Andrade et al. leg.; 2 ³ ( MZSP 64894 View Materials ), BRAZIL, Pará, Parauapebas, Caverna S 11- Cav 3 [−6.411484°, −50.334582°], 22 – 31.v. 2010, R. Andrade et al. leg; 1 ♀ (CHNUFPI 2177),
BRAZIL, Pará, Marabá, Margens da BR-155, Rodovia Augusto Montenegro [−4.413595°, −49.073536°], 7.x. 2016, L.S. Carvalho leg.
Etymology
Latin adjective lūcifer (feminine lūcifera, neuter lūciferum) (light-bringing), referring to the light-coloured pedipalpal tibiae.
Diagnosis
Femur III and IV uniformly obscure ( Figure 8 View Figure 8 (a,b)) (without lighter portions like in P. gracilis ); tibia and tarsus of pedipalps whitish ( Figure 1 View Figure 1 (d – e)), tibia with scarce and low dorsal granulation (vs coarse granulation in P. gracilis and smooth surface on P. orcus sp. nov.) ( Figure 5 View Figure 5 (a,b)); basitarsus I inflated and three times longer than each of the other tarsomeres ( Figure 4 View Figure 4 (e)).
Description
Measurements male holotype (MNRJ 2560). DSL = 3.9; DSW = 3.4; ID = 1.7; pedipalpus: coxa = 1.2, trochanter = 0.8, femur = 3.3, patella = 1.8, tibia = 1.7, tarsus = 1.3, total = 9.6; leg IV: femur = 6.8, tibia = 3.5, metatarsus = 8.6, total = 25.4.
Dorsum ( Figure 1 View Figure 1 (a–c); 3(a–c)). Anterior margin of prosoma with small scattered granules, with low anterior eminence. Interocular region with small granules and one central tubercle. Eye mound smooth. Lateral margin with row of small granules. Area I divided, with five small granules and a central tubercle on each side. Area II with about 20 small granules and area III with 18 small granules and a pair of paramedian spines. Areas I – III slightly convex. Posterior margin slightly convex with row of small granules. Free tergites I – III with row of medium granules.
Venter ( Figure 1 View Figure 1 (b)). Coxa I – III with a medial row of tubercles and coxa I – IV with scattered granules. Genital operculum with six very small granules. Free sternites with a row of small granules.
Chelicerae ( Figure 1 View Figure 1 (a–c)). Swollen. Segments I – II smooth. Mobile finger with a large basal tooth and with two small distal teeth; fixed finger with two small distal teeth.
Pedipalps ( Figure 1 View Figure 1 (dıe); 5(aıb)). Coxa dorsally with two basal medium tubercles and two large ventral tubercles. Trochanter dorsally with one small medial tubercle, one basal and one medial medium tubercle. Femur dorsally with row of minute granules and row of small ventral granules, besides a medium tubercle on sub-basal ventral portion. Patella dorsally with scattered small granules and ventrally smooth. Tibia dorsally with scattered small granules, ventrally with a row of ectal medium tubercles; tibia mesal IIiIi, ectal IIIiIi. Tarsus dorsally smooth, ventrally with a row of ectal medium tubercles; tarsus mesal IiIii, ectal Iiii.
Legs ( Figure 4 View Figure 4 (a–f)). Coxae I – III dorsally with two tubercles. Trochanter I ventrally with one basal and two apical tubercles; II – IV ventrally with a pattern of two basal, one medial and two distal tubercles. Trochanter I dorsally smooth, II – III with one retrobasal, two medial and one retroapical tubercles; IV dorsally with one retroapical high tubercle, two medium dorsal tubercles and some scattered small tubercles. Femora I – II with retroventral and proventral rows with small tubercles, others rows with minute granules; III with retroventral and proventral rows with medium tubercles, other rows with small granules; one retrodorsal and one prodorsal apical medium large tubercle; IV with retroventral and proventral rows with tubercles increasing in size distally, others rows with medium tubercles; one retrodorsal and one prodorsal apical high large tubercle. Patella I – II smooth; III with rows of small granules and a pair of dorsal small large tubercles; IV ventrally with scattered rows of small granules and a pair of conspicuous dorsodistal large tubercles. Tibia I – II smooth; III – IV with rows of minute granules, retroventral and proventral row of small tubercles. Metatarsus I – IV smooth. Basitarsus I inflated and three times longer than the other tarsus. Tarsal process reduced, with a terminal long set. Legs III – IV without tarsal scopula. Tarsal counts: 7(3)/14(3)/6/7.
Penis ( Figure 1 View Figure 1 (fıg); 7(a–c)). LP trapezoidal, with lateral sides concave, and distal border with rounded ventral tongue; LP clearly differentiated from the truncus/malleus by a ventral crack. Stylus curved, with large triangular dorsal process. MS A1 – 2 straight, positioned dorsolaterally, slightly below half of the LP; MS B absent; MS C1 – 3 being born close to the dorsomedial region, large and apically curved; MS D1 absent, MS D2 straight and large, dorsomedially located, anterior to MS A. Microsetae field restricted to lateral regions of the LP, with a large gap between them.
Colouration. Body and appendages in alcohol Dark Brown (59) and Dark Reddish Brown (44) in vivo, coxae I – IV ventrally, trochanters, chelicerae, pedipalps (except tibia) reticulated; pedipalp (except tibia) in alcohol and in vivo (brighter) Dark Brown (59), tibia in alcohol and in vivo (brighter) Yellowish White (92). Spines of area III Brownish Black (65).
Female paratype (CHNUFPI 2177): Dorsal scutum length = 3.6; dorsal scutum width = 3.2; interocular distance = 1.7; pedipalp: coxa = 0.7, trochanter = 0.5, femur = 3.1, patella = 1.6, tibia = 1.4, tarsus = 1.4, total = 16.1; leg IV: femur = 6.3, tibia 3.6, metatarsus = 7.4, total = 21.4. Similar to male, except for chelicera not swollen, legs finely granular, distitarsus I not inflated and elongated. Pedipalpal tibia mesal IIiIi, ectal IIiIi; tarsus mesal IiIi, ectal Iiii (this variation could be intraspecific and without sexual dimorphism).
Distribution
Brazil, Pará state: Canaã dos Carajás; Parauapebas; Curionópolis; Marabá.
Habitat remarks
The specimens of P. lucifer sp. nov. were registered from different geomorphological units of the Carajás Mineral Province (PMC), recognised worldwide for its enormous mineral wealth, composed of large deposits of iron, nickel, copper and gold ( Piló et al. 2015). In this ferruginous geosystem, more than 1,000 small cavities (average 30 m long) have been identified, being one of the largest concentrations of caves in Brazil ( Piló et al. 2015). Populations of P . lucifer sp. nov. are frequently found in the entry regions of these cavities and adjacent epigeal regions, which present two groups of plant typologies characteristic of ferruginous environments: steppic savanna vegetation (covering rock, shrub and deciduous nano-forests vegetation in the plateau of mountain range) and the ombrophilous forests (in the lower portions of the terrain and hillsides). In the first vegetation type, the stratum is predominantly shrub, with high density of plants. In the herbaceous stratum, there is a great diversity of grasses, orchids and other voluble plants that usually occupy the base of the rocky blocks ( Mota et al. 2015). Although most of the species present are annual, they are prominent in the rainy season but dry completely during periods of drought. Thus, the general appearance of vegetation in the dry season closely resembles the Northeast Caatingas ( Mota et al. 2015). The ombrophilous forests of this ferruginous geosystem have the smallest feature in relation to the plains forests and the remarkable presence of rocky blocks in the middle of the understory. Often these forests are associated with rocky cliffs and/or occurrence of cavities ( Mota et al. 2015). The presence of P . lucifer sp. nov. in surface and subterranean ecosystems, under different phytophysiognomic contexts, emphasise the flexibility of their populations in terms of habitat. This ecological valence can be responsible for the wide distribution of the species in PMC, being sampled in distant locations in more than 100 km. As demonstrated by Ferreira et al. (2015), the discovery of one of the largest mineral reserves in the world and the beginning of exploration in the 1960s prompted a large population exodus to the region. Such economic and social expansion generated disorderly exploitation of natural resources and degradation of the Amazon forest, mainly related to the cultivation and replacement of natural areas by pastures, due to livestock ( Ferreira et al. 2015). The advancement of mineral exploration areas also poses a threat to the populations of P . lucifer sp. nov. This type of mineral extraction promotes complete alteration of the landscape, since all the ferruginous crust that recovers the reserves of iron ore (canga) is completely removed and discarded to allow access to the mineral deposits ( Jacobi and Carmo 2008). These anthropic activities affect the different habitats of P . lucifer sp., they segregate populations and threaten the conservation of this new taxon.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Stygninae |
|
Genus |