Phymaturus rahuensis, Marín, Andrea González, Pérez, Cristian Hernán Fulvio, Minoli, Ignacio, Morando, Mariana & Avila, Luciano Javier, 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4121.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:CD5E63FC-E0FA-41B4-A2EB-ED1F66039AC5 |
|
DOI |
https://doi.org/10.5281/zenodo.5678644 |
|
persistent identifier |
https://treatment.plazi.org/id/03BCE952-2600-7000-FF7A-CCE7FCC9FCFC |
|
treatment provided by |
Plazi |
|
scientific name |
Phymaturus rahuensis |
| status |
sp. nov. |
Phymaturus rahuensis sp. nov.
( Fig. 4 View FIGURE 4 , 5 View FIGURE 5 , 6 View FIGURE 6 )
Phymaturus sp. 16 Morando, M., Avila, L.J., Perez, C.H.F., Hawkins, M. and Sites, Jr. J.W., 2013, Molecular Phylogenetics and Evolution, 66, 698.
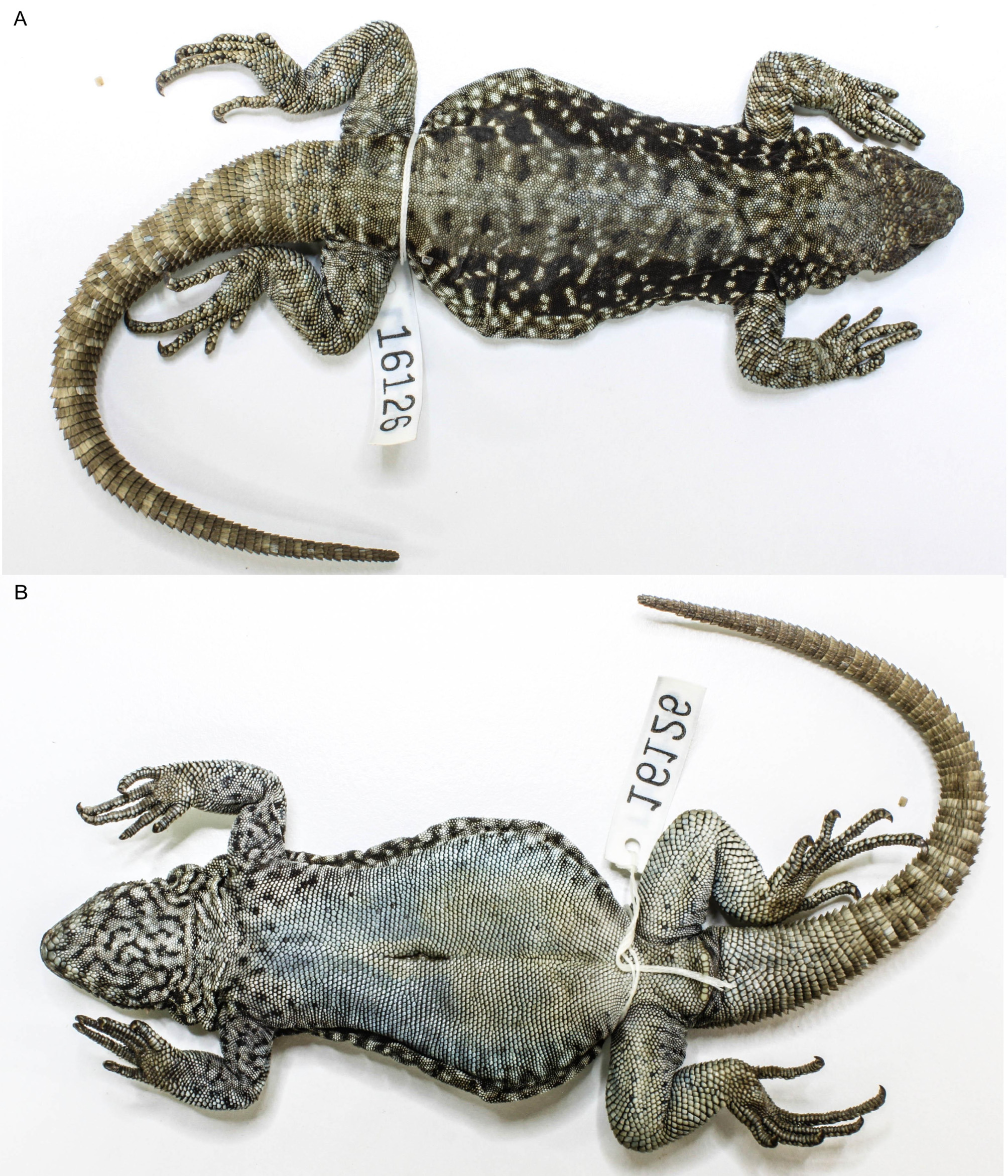
Type material. Holotype: LJAMM-CNP 16126, adult male collected on rocky hills of Provincial Road 46, 25 km E Rahue, 6 km junction Provincial Road 24, Bajada de Rahue, La Jardinera stream (39º23’15.5”S, 70º43’57.1”W, 1127 m, datum = WGS 84), Catán Lil department, Neuquén province, Argentina, A. González Marín, L.J. Avila and C.H.F. Pérez, collectors.
Paratypes: LJAMM-CNP 16117-16120, 16123, 16127 (adult males) and LJAMM-CNP 16121-16122, 16124- 16125, 16128-16129 (adult female), same data as holotype. LJAMM-CNP 5378 (adult male) and 5379 (adult female) collected on rocky cliffs on Provincial Road 46, 25 km E Rahue, 6 km junction Provincial Road 24, Bajada de Rahue, La Jardinera stream (39º23’15.5”S, 70º43’57.1”W, 1127 m, datum = WGS 84), Catán Lil department, Neuquén province, Argentina, L.J. Avila, C.H.F. Pérez, K. Dittmar, M. Morando & J.W. Sites, Jr. collectors.
Diagnosis. Phymaturus rahuensis sp. nov. is a member of the Phymaturus patagonicus group because it exhibits synapomophies found on this species’ group, bellies light-orange or pink, a set of enlarged scales projected over the auditory meatus and external margins of postmental scales dark pigmented (sensu Lobo et al. 2012b) and (sensu Etheridge 1995) flat imbricate superciliaries, non-rugose dorsal scales on tail, and subocular scale usually not fragmented. Within the Phymaturus patagonicus group, P. rahuensis sp. nov., differs from P. cacivioi , P. castillensis , P. ceii , P. desuetus , P. excelsus , P. felixi , P. indistictus , P. manuelae , P. spectabilis , P. spurcus , P. tenebrosus , P. videlai , in the presence of a dorsal pattern formed by dispersed white spots. Scales around midbody are non-overlapping among P. rahuensis (216–261), P. patagonicus (162–192) and P. yachanana (160–194). Number of ventral scales in P. rahuensis (167–193) is higher and slightly overlapping with P. patagonicus (142– 171) and P. yachanana (135–168). Average number of scales around midbody in P. rahuensis (X̅= 236.7) is higher than in P. somuncurensis (X̅= 218.29), P. sinervoi (X̅= 227.9), P. calcogaster (X̅= 221.20), P. etheridgei (X̅= 221.55) and with P. camilae (range 205-228, Scolaro et al. 2013). Phymaturus rahuensis sp. nov. has a marked sexual dichromatism not present in P. nevadoi (according to original description) and P. sitesi . Phymaturus rahuensis sp. nov. has a dorsal pattern coloration (well marked in females) formed by a paravertebral band with two dorsolateral series of quadrangular to irregular marks fused to the paravertebral band but usually separated each other by dark lines, a pattern not observed in any other species of the clade. Phymaturus rahuensis sp. nov. has a dorsal coloration with small rounded white spots scattered on its body (4–12 scales size each) similar to P. nevadoi (4–9 scales) and P. delheyi (1–10 scales), larger than in P. sitesi (1–2 scales), but smaller than in P. payuniae (4–40 scales, sometimes fused forming irregular marks) and P. zapalensis (5–14 scales). Phymaturus rahuensis sp. nov. has a tail with vanishing rings as P. zapalensis but not spotted as in P. delheyi , P. nevadoi , P. payuniae , and P. sitesi . Average scales around midbody number is higher in Phymaturus rahuensis sp. nov. (X̅= 236.7, SD = 12.8) than all other species of the group: P. nevadoi (X̅= 208.3, SD = 8.7), P. payuniae (X̅= 219.0, SD = 11.7), P. sitesi (X̅= 221.5, SD = 7.9), P. delheyi (X̅= 216.7, SD = 15.7) and P. zapalensis (X̅= 221.2, SD = 13.8). Average ventral scale number is lower in P. rahuensis (X̅= 179.6, SD = 7.2) than in P. payuniae (X̅= 183.3, SD = 10.4), P. delheyi (X̅= 185.5, SD = 9.2) and P. zapalensis (X̅= 186.3, SD = 13.0) but higher than P. nevadoi (X̅= 164.0, SD = 8.4) and P. sitesi (X̅= 176.2, SD = 9.5). Average supralabials scales number is lower in P. rahuensis (X = 8.0, SD = 0.5) than in the other species of the group: P. nevadoi (X̅= 9.2, SD = 0.5), P. payuniae (X̅= 11.1, SD = 1.1), P. sitesi (X̅= 9.2, SD = 1.2), P. delheyi (X̅= 9.0, SD = 0.8) and P. zapalensis (X̅= 8.8, SD = 0.8). Phymaturus rahuensis sp. nov. has lower finger lamellae number (X̅= 19.8, SD = 1.1) than the P. nevadoi (X̅= 22.6, SD = 1.4), P. payuniae (X̅= 22.1, SD = 1.1), P. sitesi (X̅= 21.8, SD = 0.9), P. delheyi (X̅= 22.4, SD = 1.1) and P. zapalensis (X̅= 21.7, SD = 1.8) and lower toe lamellae number (X̅= 25.8, SD =1.1) vs P. nevadoi (X̅= 28.8 SD = 1.2), P. payuniae (X̅= 27.8, SD = 1.9), P. sitesi (X̅= 28.8, SD = 1.2), P. delheyi (X̅= 29.6, SD = 2.0) and P. zapalensis (X = 28.4, SD = 1.8). The new species can be distinguished from P. nevadoi , P. payuniae , P. zapalensis , P. delheyi and P. sitesi by differences in mitochondrial and nuclear genes (exclusive haplotypes with cytochrome b and 12S mitochondrial genes and with PNN, Phy38 and Phy41 nuclear markers ( Morando et al. 2013). Based on cyt-b data ( Morando et al., 2013), uncorrected genetic distances between previously morphologically described species of Phymaturus are low (e.g. for a species pair within the patagonicus group: P. nevadoi vs. P. payuniae = 0.81%, in Avila et al. 2014); some species pairs even have smaller values, but they may be conspecific ( Morando et al., 2013). The smallest uncorrected genetic distances between P. rahuensis sp. nov. and other taxa of the payuniae clade are: P. rahuensis vs P. zapalensis = 1.7%, P. rahuensis vs P. sp. 17 = 1.44%, while with the rest of the species of this clade, genetic distances are> 3.6%.
Description of the holotype. Adult male 88.1 mm (SVL). Axilla-groin distance 45.5 mm. Tail length 101.9 mm. Head length 15.5 mm; head width 16.8 mm; head depth 9.4 mm; snout length 5.6 mm (from anterior border of the orbit to tip of snout), horizontal diameter of the orbit 6.6 mm. Arm length 26.5 mm; tibial length 17.9 mm; foot length 24.4 mm. Upper head scales smooth, convex, bulged, pitted with scale organs in postrostral, internasals, frontonasals, frontals, and prefrontals. Rostral flat, two times wide as long (2.7 x 1.1 mm). Two postrostrals on the left side, the outermost smaller than the inner, in the right side abnormally small and numerous, with conspicuous scale organs (1-6); rostral and nasal not in contact, separated by the anterior lorilabial scale and a small prenasal.
Nasal scales almost rounded (1.3 x 1.5 mm). Nostril rounded, occupying almost all the nasal scale. Nasal scales in contact with 9-8 scales. Internasal, frontonasals, medium in size, irregular in shape; prefrontals and frontals, medium in size, hexagonal; supraorbital semicircles complete, 13–11 enlarged supraoculars; frontoparietals, parietals, and circumorbitals irregular in size and shape from each other. Interparietal hexagonal, only distinguishable by a large and conspicuous white cream “eye” in the middle, occupying most of the scale. Eighteen dorsal head scales between rostral and nuchals. Two scales between nasal and first canthal. First canthal small, higher than wide, convex with two scale organs. Posterior canthal larger, longer than wide. Posterior canthal slightly overlaps first supercilliary. Supercilliaries 7–5 (left-right) irregular flattened and elongated, on left sides overlapped the first five, and the right side the three first scales. Loreal region flat, three irregular scales on both sides. Upper ciliary scales in two rows, those of inner rows flat and quadrangular, those of outer row granular and compressed. Lower and upper ciliaries similar in size and shape. Palpebral scales small, irregular, slightly granular. One preocular, small, square; one elongate subocular (4.5 x 0.6 mm), unfragmented, one small postocular; a well marked longitudinal ridge along upper margin of subocular and preocular scale, and not marked in postocular. Two rows of lorilabials becoming only one in half subocular. Lorilabials convex, 12/6–10/6, approximately rectangular, slightly narrow than supralabials, pitted with 1–5 scale organs. Supralabials 7, flat. Temporal scales conical, smooth, swollen, juxtaposed, with a scale organ at the inferior side. Auditory meatus higher than wide (4.1 x 2.0 mm) with 3 outwardly projecting scales along anterior border; posterior border surrounded by granular scales. Mental pentagonal, wider than long (2.2 x 1.4); in contact with anterior infralabials, anterior sublabials and postmentals. Infralabials 6. Chinshields 5-4, irregular, slightly quadrangular, separated from infralabials by series of 1–2–3 irregular scales, first equal in size but becoming smaller to back. One-4 scale organ present in some supralabials and infralabials. Gular scales round, flat, and juxtaposed. Sixty-six between auditory meatus. Antehumeral, gular and lateral fold well developed, antegular fold notorious, and postauricular folds few developed. Ante-humeral pocket well developed. Fifty-seven scales between auditory meatus and scapula. In ventral view, gular fold incomplete with its anterior margins delimited with enlarged scales on their borders. Dorsal body scales rounded, smooth, juxtaposed. Forty-four dorsal scales along midline of the trunk in a distance equivalent to head length. Scales around midbody 216. Scales on flanks conical. Ventral scales larger that dorsals, rhombals and moderately imbricated. Ventral scales between mental and precloacal pores 178. Scales of the cloacal apron equal in size than body scales, flat, rounded, moderately imbricate. Precloacal pores eight. Supra-brachial and antebrachial scales smooth, rhombals and imbricated, larger than dorsal body scales. Supracarpals laminar, rhombals, smooth. Supradigital lamellae convex, imbricate. Infra-brachial and ante-brachial scales small, granular. Subdigital finger lamellae with 3–4 keels (more conspicuous in proximal lamellae), 3–4 mucronate. Subdigital finger lamellae numbering I: 11; II: 15; III: 19; IV: 21; V: 15. Claws moderately long. Infracarpals with round margins and 1-3 obtuse keels, 1-3 mucronate. Supra-femorals smooth, imbricate, rhomboidal to round. Infra-femorals slightly larger and imbricate, smooth, rhomboidal. Supra-tarsals rhombals, smooth and imbricate. Post-femoral scales smaller and granular. Supra-tibials imbricate, conical, and mucronate. Infra-tibials larger than infra-femorals, rhombals, smooth, imbricate. Infra-tarsals with 1–3 obtuse keels, mucronate. Sub-digital toe lamellae numbering I: 12; II: 16; III: 22; IV: 27; V: 18. Caudal scales arranged in spinose annuli, scales larger than body and limbs scales, slightly keeled, imbricated, out-projecting.
Holotype coloration. Dorsal background coloration brown/dark drab, more intense on trunk becoming cinnamon-drab on head, limbs, and tail. Dorsal coloration pattern on body is a wide dorsal band with a clear and continuous vertebral band and two dorsolateral series of irregular rounded to roughly quadrangular spot wide joined to the vertebral band and separated each other by a narrow dark transversal line that disappear in some areas becoming like dark irregular small spots along paravertebral line sides. On each side of the trunk, between neck to anterior insertion of the hind limbs two conspicuous dusky brown/black lateral bands. Trunk, forelimbs and head speckled with cream white (4–12 scales) more evident on lateral and dorsolateral bands becoming less conspicuous on head and forelimbs and almost not evident on vertebral band and neck. Limbs with cream white reticulate. First two thirds of the tail with cinnamon-drab vanishing rings becoming non-conspicuous in the distal portion. Throat, gular region, malar region, neck lateral region and upper chest pale neutral grey with dusky brown reticulate. Lower chest and upper belly yellow ocher, more intense in cloacal apron and adjacent femoral region. Ventrolateral region drab with dusky brown reticulated. Ventral region of anterior forelimbs pale neutral grey and posterior forelimbs cream white. Ventral region of tail pale neutral grey.
Color in preservative. After preservation, head, dorsum, body flanks and tail maintain original coloration but darken and lose some tones. Chest, belly and femoral regions lost the distinctive ventral yellow-ocher.
Variation. Based on eight adult males ( Table 4, Fig. 4 View FIGURE 4 , 6 View FIGURE 6 ): SVL 83.6–88.11 mm. Axilla-groin distance 38.9– 45.5 mm. Foot length 21.8–24.4 mm. Tibial length 15.5–18.5 mm. Arm length 24.4–26.8 mm. Head length 15.1– 15.9 mm. Head width 15.0– 16.8 mm. Head height 9.0– 9.5 mm. Midbody scales 216–261. Dorsal scales in head length 44–49. Ventral scales 167–186. Supralabials 7–9. Infralabials 6–9. Scales around nasal 6–8. Third finger lamellae 18–22. Fourth toe lamellae 24–28. Precloacal pores 8–11. In seven adult females ( Table 4, Fig. 5 View FIGURE 5 , 6 View FIGURE 6 ): SVL 72.2–95.1 mm. Axilla-groin distance 37.6–51.0 mm. Foot length 21.6–23.0 mm. Tibial length 15.2–17.6 mm. Arm length 23.3–25.0 mm. Head length 13.0– 15.9 mm. Head width 13.8–16.6 mm. Head height 7.4–9.4 mm. Midbody scales 221–244. Dorsal scales in head length 40–46. Ventral scales 176–193. Supralabials 8–9. Infralabials 7–8. Scales around nasal 6–8. Third finger lamellae 19–21. Fourth toe lamellae 25–27. Coloration in females differs from the males but follow the same coloration pattern but it is more noticeable, with larger dorsolateral spots (10– 12), and well distinct. Dorsal band is very distinctive from the lateral band. In female LJAMM-CNP 16128, dorsal background of body, head, forelimbs and tail is beige. In same females, dorsolateral spots are smaller and irregular, from rounded to like a four point star, then transversal separating lines expand its size becoming rounded along the vertebral band giving the appearance of a series of dark ocelli. Lateral region of body, from the neck region to the groin, dusky brown/black, speckled with cream color smudges (5–30 scales), postauricular region only reticulated. Dorsal pattern of limbs with white cream reticulate and a few scattered dusky brown spots (1-2 scales). Tail with white cream reticulate, transforming in rings toward the posterior half region. Ventral surface pale neutral grey. Throat, gular region, malar region and neck lateral region with dusky brown reticulate. Lower belly, cloacal apron, femoral, tibial and tarsal region orange yellow. Ventrolateral region pale neutral grey with dusky brown reticulated.
Ventral tail beige. The other female of the type series has the same color pattern. Eight males have the same basic color pattern with greater or lesser degree of development of the dorsal cream white spots or extension of the dusky brown area. In the specimen with completely dusky brown dorsum, the spots in the dorsolateral region are denser. In some specimens in the dorsal band, the spots are absent and in other, the dusky brown spots form transverse stripes. In males the yellow ocher color in the lower belly, cloacal and femoral adjacent region is similar in all the specimens but LJAMM 16127 that also has an orange yellow color in the first third of the tail.
Males (n= 8) Females (n= 7)
Mean SD Range Mean SD Range
SVL 85.6 1.7 83.6–88.11 86.8 8.0 72.2–95.1 AGD 42.3 2.3 38.9–45.5 44.9 4.5 37.6–51.0 HL 15.3 0.2 15.1–15.9 14.8 1.0 13.0–15.9 HW 16.0 0.5 15.0–16.8 15.3 0.9 13.8–16.6 HH 9.3 0.2 9.0–9.5 8.7 0.7 7.4–9.4
FL 23.2 0.9 21.8–24.4 22.4 0.4 21.6–23.0 TL 17.2 0.8 15.5–18.5 16.6 0.9 15.2–17.6 AL 25.4 0.9 24.4–26.8 24.1 0.5 23.3–25.0 SAMB 240.6 15.5 216–261 232.2 7.8 221–244 DS 46.3 1.8 44–49 42.8 2.6 40–46
VS 177 7.2 167–186 182.5 6.6 176–193 LM 20 1.5 18–22 19.7 0.7 19–21
LP 26 1.3 24–28 25.7 0.9 25–27
SLS 7.8 0.6 7–9 8.1 0.3 8–9
ILS 7.6 0.9 6–9 7.5 0.5 7–8
PC 9.3 0.9 8–11 – – –
Etymology. The specific name, “ rahuensis ”, refers to the region where the species was collected "Bajada del Rahue". The Rahue toponym in Mapudungun means "place where there is gray clay".
Geographic distribution. Phymaturus rahuensis sp. nov. was only collected on rocks 1127 m above sea level in Bajada del Rahue at La Jardinera streams, in the mountain range formation known as Catán Lil mountains. This location has rocky marine sediments from middle Jurassic; the area was occupied by marine ingression and regression that deposited the sedimentary landscape, named as Los Molles Formation ( Riccardi 1993; García Morabito 2010). All range was affected by Holocenic glaciations but details about its effects on the landscape (and the biota) are still unknown ( Rabassa et al. 2011) ( Fig. 7 View FIGURE 7 ).
Natural history. Phymaturus rahuensis sp. nov. is a common and easy to observe species. Little information about natural history and biology of this new species is available. Phymaturus rahuensis sp. nov. was usually found surrounded by vegetation characteristic of the Patagonica Phytogeographic Province, Payunia District, dominated by communities of Senna arnottiana and Stillingia patagonica inter alia ( Roig 1998), also species as Haploppapus sp., Mulinum spinosum and several species of grasses ( Stipa spp. and Festuca spp.) were observed. The holotype and paratypes were found by active search, but usually they were spotted basking on rocky outcrops or hiding in crevices. Liolaemus elongatus and L. kriegi are the only observed lizards that shared the same microhabitat. One species of amphibian, Pleurodema bufoninum , was observed on a little stream. No data about reproduction, diet or other natural history characteristics are available, but as in other related species of Phymaturus , Phymaturus rahuensis sp. nov. probably is viviparous and feeds on plant matter, and possibly some arthropods.
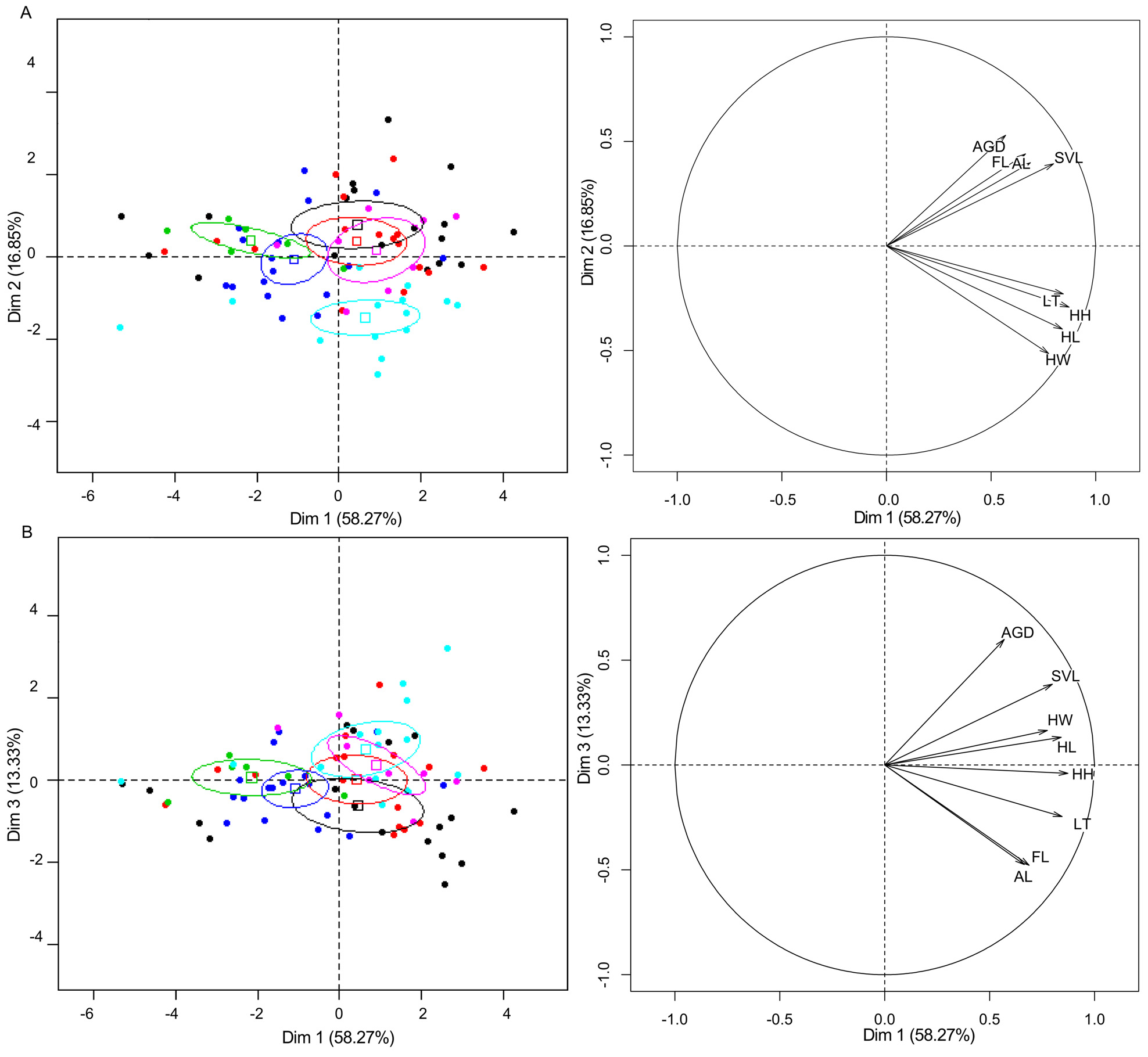
Discussion. The aim of this study was to test the previously molecularly based proposed hypothesis of candidate species for P. sp. 16, by implementing two additional sources of independent methods within the context of the integrative taxonomy. Considering the evidence of these three methodological approaches (molecular analyses, linear and geometric morphometric), and based on the convergent results of these methods, we now consider this hypothesis as a valid species. Therefore, the taxon described above as Phymaturus rahuensis sp. nov. corresponds to populations previously called P. sp. 16 in the recent molecular phylogenetic study of Morando et al. (2013). This new taxa with P. nevadoi , P. payuniae , P. zapalensis , P. delheyi and P. sitesi , and two other candidate species (P. sp. 12, P. sp. 17), belong to the payuniae clade, which had strong support on the BEST all genes species tree ( Fig. 6 View FIGURE 6 in Morando et al. 2013, pp=1). Using characters of several sources but mainly morphological characters, Lobo & Nenda (2015) found P. nevadoi , P. payuniae , P. d e l h e yi and P. sitesi , as a monophyletic group but P. zapalensis was inferred as closely related to P. tenebrosus ( Fig. 5 View FIGURE 5 in Lobo & Nenda 2015). Almost all of these species have been described based on external descriptive morphology and coloration, and only some of them were coupled with supposed geographical isolation and molecular differences (Avila et al. 2011; 2014). Diagnoses of new taxa in the context of traditional taxonomy usually employ only summary statistics as the basis for describing new species ( Ocampo et al. 2012), with these type of data values clearly overlap and sometimes are useless to detect morphometric differences among most of the species of Phymaturus . In recent years, multivariate methods, e.g. the principal component analysis (PCA) has been used to differentiate and describe species of Liolaemus and Phymaturus (e.g. Breitman et al. 2011a; 2011b; Aguilar et al. 2013; Avila et al. 2014). This multivariate analysis is helpful to understand what variables contribute most to the morphological variation, as well as the interactions among them ( Claude 2008; Abdi et al. 2013; Minoli et al. 2014). In our results, Phymaturus rahuensis sp. nov. was completely differentiated from all other described species ( Fig. 2 View FIGURE 2 A, B). Thus similarly to other species of Liolaemini (see Aguilar et al. 2013; Avila et al. 2014; Breitman et al. 2011a; 2011b; Scolaro et al. 2013), this analysis showed to be useful to differentiate closely related species. The geometric morphometric approach (MG) is a tool useful to capture variation in form and provides a solid statistical framework for studying the geometric properties of the analyzed structures ( Bookstein 1991; Adams et al. 2004; Kaliontzopoulou 2011). This method proved to be a powerful tool to capture shape variation among closely related species of lizards (e.g. Florio et al. 2012; Kaliontzopoulou et al. 2012). Our results of geometric morphometric analyses showed Phymaturus rahuensis sp. nov. completely differentiated from other described species ( Fig. 3 View FIGURE 3 A, B), same results were obtained using principal component analysis. Following our operational criterion, we consider two taxa as different if they present evidence of significant differences with both classic (linear measurements and scales) and geometric morphometrics (differences in shape). These two different morphological approaches coupled with previously published molecular results, support the distinction of P. sp. 16 that formerly was proposed as a candidate species ( Morando et al. 2013). Within the integrative taxonomy framework, the species hypotheses endorsed with data from different sources of information have a higher level of support than those sustained by a single type of evidence ( Dayrat 2005; Padial & de la Riva 2007). The complexity of the evolutionary process in a lineage or species requires that the taxa limits should be studied from multiple complementary perspectives, to integrate them into a standardized framework for evaluating those boundaries (Padial & De la Riva 2007). This framework can be usefully applied within a speciose lineage such as Liolaemini (e.g., Aguilar et al. 2013; Minoli et al. 2014), and could significantly improve taxonomic stability.
| LJAMM-CNP |
Centro Nacional Patagonico (CENPAT-CONICET) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |