Phyllium (Phyllium) jacobsoni Rehn & Rehn, 1933
|
publication ID |
https://doi.org/10.11646/zootaxa.2322.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/4C724261-6C54-3A78-FF39-FD51301FC27F |
|
treatment provided by |
Felipe |
|
scientific name |
Phyllium (Phyllium) jacobsoni Rehn & Rehn, 1933 |
| status |
|
Phyllium (Phyllium) jacobsoni Rehn & Rehn, 1933 View in CoL
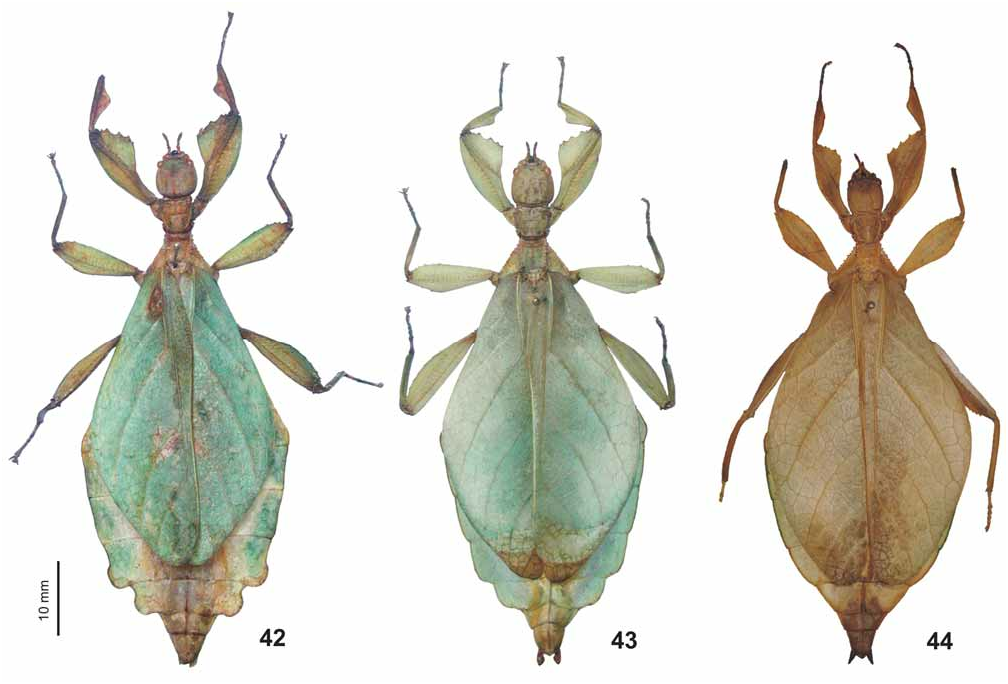
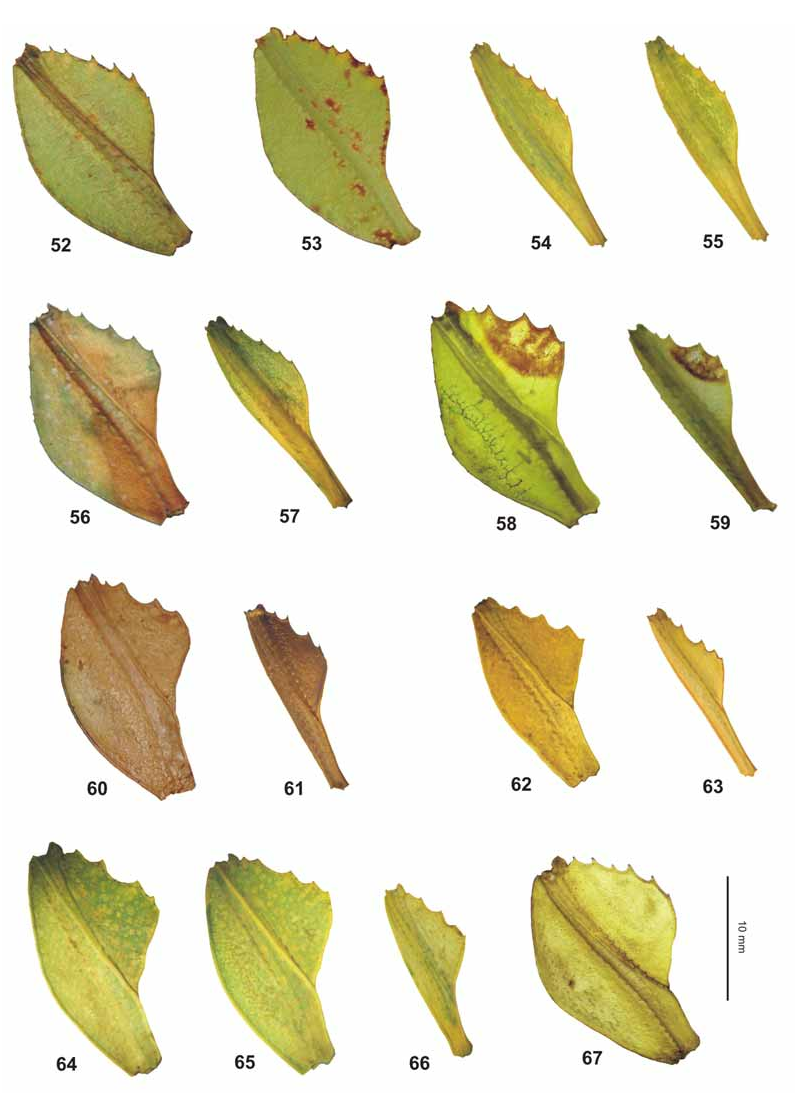
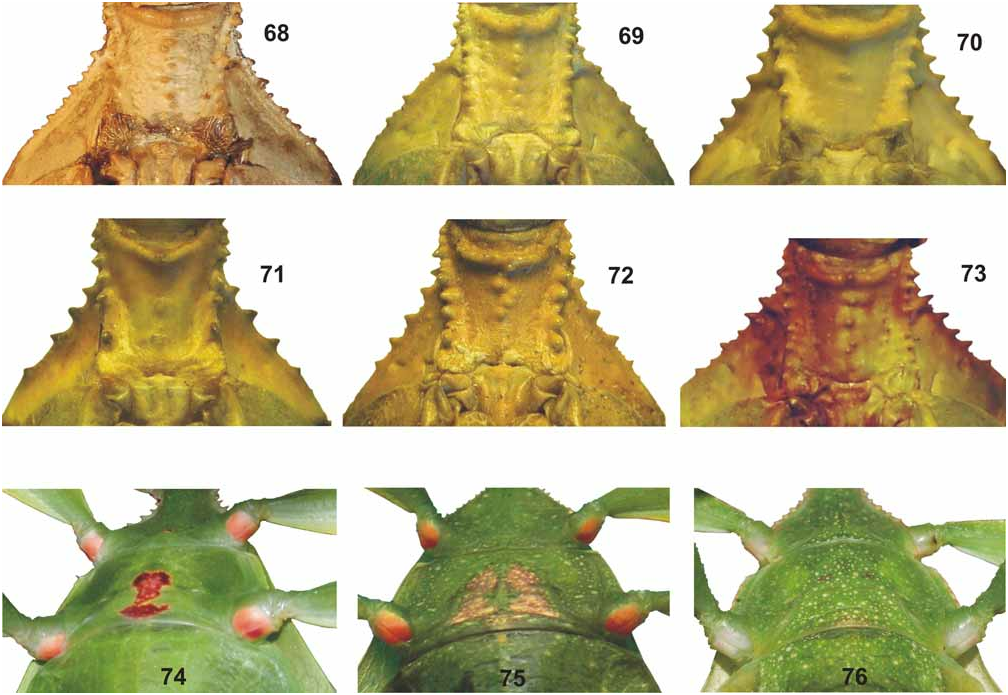
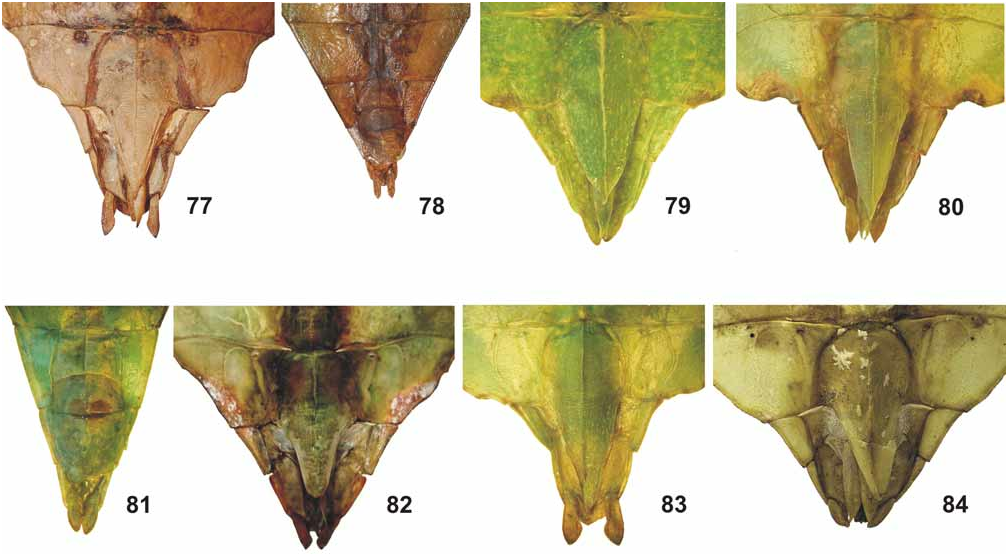
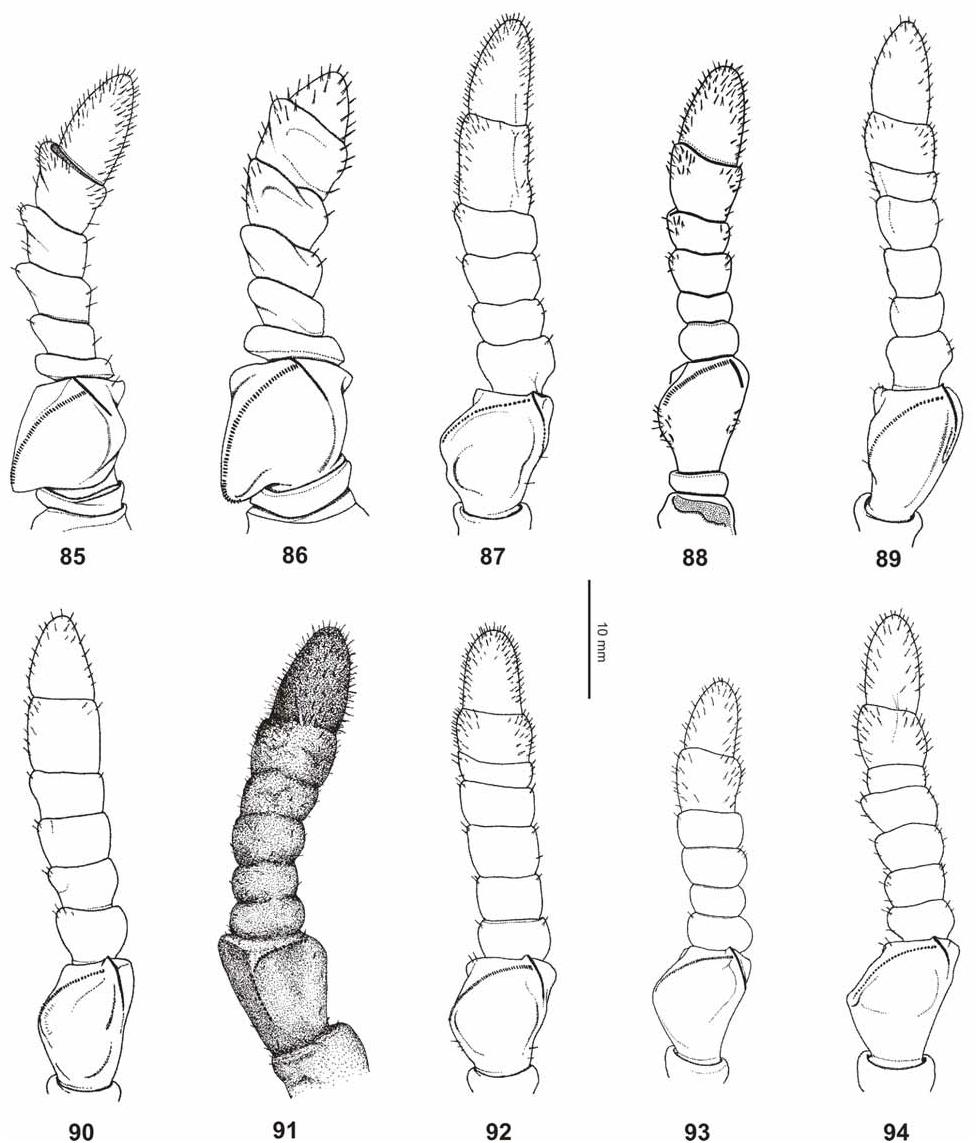
( Figs. 42–44 View FIGURES 42–44 , 62–63 View FIGURES 52–67 , 76 View FIGURES 68–76 , 83 View FIGURES 77–84 , 93 View FIGURES 85–94 , 107–108, 117)
Phyllium jacobsoni Rehn & Rehn, 1933: 419 View in CoL , pl. 17: 7 ( ♀). HT, ♀: E. Jacobson, Java, Jan. 1911, Nongkodjadjar; Phyllium jacobsoni Rehn & Rehn View in CoL , TYPE (ANSP), Type No. 5535; AT, ♂: ♀: E. Jacobson, Java, Jan. 1911, Nongkodjadjar;
Phyllium jacobsoni Rehn & Rehn View in CoL , PARATYPE, Allotype ♂ (ANSP). Klante, 1976: 70. Grösser, 2001: 84, fig. 111. Zompro & Grösser, 2003: 136. Otte & Brock, 2005: 274. Grösser, 2008: 122, fig. 145 ( ♀).
Phyllium bilobatum, Redtenbacher, 1906: 177 View in CoL (in part – all specimens from Java, Malang). Klante, 1976: 71 (in part – only specimens from Java).
Phyllium geryon, Griffini, 1898a: 11 View in CoL . Griffini, 1898b: 2, figs. A, B ( ♂, ♀). Redtenbacher, 1908: 177, pl. 6: 12 ( ♂) (in part – only specimens from Java). Giglio-Tos, 1910: 16. Rehn, 1912: 126. Caudell, 1927: 19.
Phyllium siccifolium, Redtenbacher, 1906: 176 View in CoL (in part – all records from Java).
Material examined [ 20 ♀, 12 ♂♂, 15 nymphs, eggs]: JAVA: 3 ♀, 4 ♂♂, 20 eggs: Indonesien, W-Java, auf Papaya-Bäumen ( Carica papaya L.), III.–IV.2008, via Thorsten Thron; Phyllium (Ph.) jacobsoni Rehn & Rehn, 1933 (coll. FH, No’s 0637-1 to 7 & E) ; 1 ♀, 1 ♂: ex Zucht: Thorsten Thron 2008, Herkunft: W-Java, F1- Generation (coll. FH, No’s 0637-8 & 9) ; 3 ♀: ex Zucht: Thorsten Thron 2009, Herkunft : W-Java, F1- Generation (coll. FH, No’s 0637-10 to 12) ; 1 ♀: Java, Jakarta, 6.-9.2000 (coll. OC) ; 1 ♀, 2 ♂♂: Zucht Th. Thron, 2008 (coll. OC) ; 1 ♀: Java occident. Pengalengan 4000’ 1893, H. Fruhstorfer; Phyllium siccifolium L . ♀ Brunner det. (11). ( MNHU) ; 1 ♂: 6176; 6176. Java Dohrn; Phyllium geryon Gray ♂ Brunner det. (5). ( MNHU) ; 1 ♂: Java occident. Sukabumi 2000’ 1893, H. Fruhstorfer; Phyllium geryon Gray ♂ Brunner det. (2). ( MNHU) ; 1 ♀ (penultimate instar): Buitenzorg auf Java, Volkens ( MNHU) ; 1 ♀ (penultimate instar): Ostjava , Tengger Geb. 4000’, Fruhstorfer S.; Phyllium bilobatum Gray ♀ Brunner det. ( MNHU) ; 1 ♀ (nymph n4), 3 ♂♂ (penultimate instar), 2 ♂♂ (nymphs n4), 3 ♂♂ (nymphs n2), 1 nymph (newly hatched): Ostjava , Tengger Geb. 4000’, Fruhstorfer S.; Phyllium siccifolium L. Larve Brunner det. ( MNHU) ; 1 ♀ (nymph n5), 1 ♀ (nymph n4): Java occident. Pengalengan 4000’ 1893, H. Fruhstorfer; Phyllium siccifolium L . ♀ Larve Brunner det. ( MNHU) ; 1 ♂, 1 ♀: Java; Phyllium siccifolium? Gray , Java ( MIZT) ; 1 ♀: Java; Phyllium geryon ♀ Gray, Java (viaggio De Filippi) ( MIZT) ; 1 ♀: Java; Coll. Giglio-Tos, Museo di Zoologia Università Torino ( MIZT) ; 1 ♀: Coll. Br. v. W., Java occident.; det. Redtenb. Phyllium siccifolium ; 18.489 ( NHMW, No. 296) ; 2 ♀: Coll. Br. v. W., Java occident.; det. Redtenb. Phyllium siccifolium ; 18.489 ( NHMW, No. 296) ; 1 ♂ (penultimate instar): Coll. Br. v. W., Java, Buitenzorg, det. Redtenb. Phyllium siccifolium ; 20.060 ( NHMW, No. 296) ; 1 ♀: Coll. Br. Vv. W., Java, Malang; det. Redtenb. Phyllium bilobatum ; 20.537 ( NHMW, No. 298) ; 1 ♀: Mus. Caes. Vind. , Java, 1883, ida Pfeiffer; det. Redtenb. Phyllium bilobatum ( NHMW, No. 298) ; 1 ♂: Coll. Br. v. W., Java, Malang , Staud.; det. Redtenb. Phyllium geryon Gray ; 20.536 ( NHMW, No. 297) ; 1 ♂: Baron Warsberg, Java, 1868; Mus. Caes. Vind. , Java, Baron Warsberg 1868; det. Phyllium geryon Gray ( NHMW, No. 297) ; 1 ♀, several eggs: Java (coll. MG) .
NO DATA: 1 ♀: no data ( MNHU) .
Differentiation: This species shows a great deal of variation concerning to the shape of the abdomen in ♀. Virtually it is very similar and obviously closely related to the Philippine Ph. bilobatum Gray, 1843 and Malayan Ph. hausleithneri Brock, 1999 . From the first species ♀ differ by: the narrower exterior lobe and much smaller, less acute teeth of the interior lobe of the profemora ( Fig. 62 View FIGURES 52–67 ); more slender mesofemora; relatively shorter and broader meso-praescutum; much less distinct lateral spines of the mesopleurae, and broader more widely rounded lobes of abdominal segments VII and VIII.
From Ph. hausleithneri it is distinguished by: the considerably smaller size; shorter and broader mesonotum and pale pink instead of blue interior marking of the meso- and metacoxae of both sexes ( Fig. 76 View FIGURES 68–76 ). ♀ furthermore differ by: the more distinctly granulose vertex; differently structured antennae which consist of only nine segments ( 10 in hausleithneri , Fig. 93 View FIGURES 85–94 ); only 40 teeth on the pars stridens of antennomere III ( 44– 48 in hausleithneri ); more distinct teeth of the interior lobe of the profemora and relatively larger cerci. ♂♂ may also be distinguished by: the longer profemora which are about 2.3x longer than the head (hardly 2x longer in hausleithneri ); very slender exterior extension and smaller interior lobe of the profemora ( Fig. 63 View FIGURES 52–67 ); much more slender mesofemora; considerably longer and more slender protarsi and slightly longer antennae. The eggs differ from those of Ph. hausleithneri by the smaller dimensions and relatively shorter and smaller micropylar plate (Figs. 107–108).
Variation: Captive breeding from a single culture-stock originating in western Java has shown this species to be strongly variable concerning to the shape of abdominal segments VII and VIII of ♀. These range from being just weakly rounded (e.g. the HT or specimen in Fig. 44 View FIGURES 42–44 ) to distinctly and roundly or ± acutely lobed ( Figs. 42–43 View FIGURES 42–44 ). While a ♀ in the second author’s collection (coll. OC, Fig. 44 View FIGURES 42–44 ) matches almost perfectly with the HT, which represents one extreme of the range, a ♀ in the first author’s collection (coll. FH No. 0637-11) has the lobes of abdominal segment extending not only posteriorly but also laterally ( Fig. 42 View FIGURES 42–44 ). The shape of the abdomen also shows slight variation in ♂♂, the AT having it elliptical to fusiform with segment IV widely rounded, while it is more spear-shaped with segment IV ± angulate in most of the captive reared specimens at hand. Some variation is also seen in the shape and size of the teeth of the interior lobe of the profemora and number of spines and tubercles on the mesothorax of both sexes. While ♂♂ are mostly plain green but may have the apical portion of the mesofemora brown, ♀ range from pale to dull green and are to a variable degree furnished with brown markings and speckles. More rarely yellow specimens may occur. Body lengths (according to Klante, 1976: 71): ♂♂ 43.0–57.0 mm, ♀ 62.0–71.0 mm. Further detailed measurements are presented in table 8 below .
Comments: As shown here, ♀ of Ph. jacobsoni show considerable variation concerning to the shape of abdominal segments VII and VIII, which has caused confusion with the Philippine Phyllium bilobatum Gray, 1843 and Malayan Ph. hausleithneri Brock, 1999 . Rehn & Rehn (1933: 419) originally described Ph. jacobsoni from a ♀ and ♂ from Nongkodjadjar in western Java, the ♀ HT having abdominal segment VII and VIII just weakly rounded.
Klante (1976: 70) recorded Javanese material from the collections of MNHU and ZMUH, which he stated to have been misidentified as Ph. geryon Gray, 1843 by Redtenbacher (1906: 177), a species originally described from the Philippines. Also the Javanese material erroneously referred to Ph. geryon by Griffini (1898b: 2, figs. A, B), Giglio-Tos (1910: 16), Rehn (1912: 126) and Caudell (1927: 27) was correctly assigned to Ph. jacobsoni by Klante (1976: 70), which was confirmed by examination of the corresponding specimens in ANSP, MIZT and USNM. All Javanese ♀ with lobed abdominal segments VII and VIII (and corresponding ♂♂ from the same localities) were attributed to Ph. bilobatum by Redtenbacher (1906: 177) and Klante (1976: 71 ff.), the latter author however expressed doubt if these Javanese specimens were conspecific with the Philippine Ph. bilobatum and furthermore stated he was unable to distinguish the ♂♂ from those of Ph. jacobsoni . Hence, Klante (1976: 71) suggested they could be different from Ph. bilobatum and might instead be conspecific with Ph. jacobsoni . Indeed, all Javanese records of Ph. bilobatum by Redtenbacher (1906: 177) and Klante (1976: 71) relate to Ph. jacobsoni . This also concerns to the Javanese specimens illustrated as “ Ph. bilobatum ” by Grösser (2008: 91, fig. 108 left). In all, Redtenbacher (1906) recorded specimens of Ph. jacobsoni from Java under three different names.
According to the numerous specimens recorded throughout European museum collections, Ph. jacobsoni appears to be rather common almost all over Java. Live adults were imported to Germany by Thorsten Thron (Gorxheimertal) in 2008 and eggs distributed to several other breeders throughout Europe. The original specimens were said to have been found exclusively on papaya-trees ( Carica papaya , Anarcardiaceae) but directly accepted oak ( Quercus robur & Q. ilex , Fagaceae ) and bramble ( Rubus fruticosus , Rosaceae ) as alternative food-plants. Captive breeding in Europe has since proven moderately easy and shown ♀ to be strongly variable concerning to the shape of the abdomen (see above). Furthermore, when threatened ♀ spray a liquid secretion from their prothoracic defence glands. Also Ph. westwoodii Wood-Mason, 1875 is known to spray a liquid secretion, but interestingly the secretion of Ph. jacobsoni has different characteristics, being transparent (whitish in westwoodii ) and almost odourless (strongly aromatic in westwoodii ).
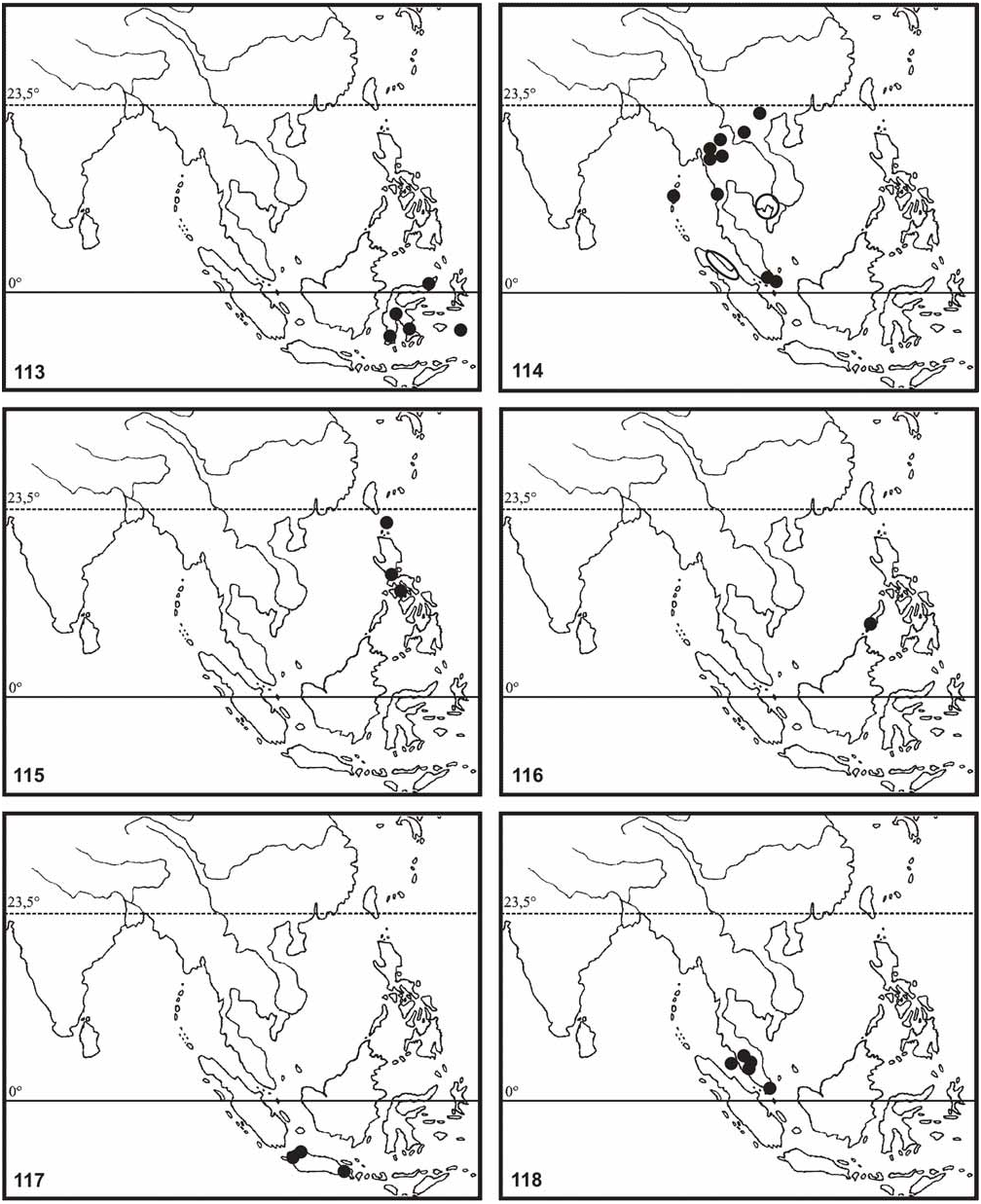
Distribution ( Fig. 117 View FIGURES 113–118 ): Java (Nongkodjadjar [type-locality], Pengalengan 4.000 ft. [MNHU; ETHZ]; Sukabumi 2.000 ft. [MNHU]; Wijnkoops-Bay [ Klante, 1976: 71 as bilobatum ]); Malang [NHMW; MNHN]; “Buitenzorg” (= Bogor) [NHMW; Caudell, 1927: 19 as geryon ]; Tijbodas, Mount Gede [ Caudell, 1927: 19 as geryon ] & Tengger Mountains 4.000 ft. [MNHU]). Believed to be endemic.
* according to Rehn & Rehn (1933: 423)
| OC |
Oberlin College |
| MIZT |
Universita di Torino |
| NHMW |
Naturhistorisches Museum, Wien |
| MG |
Museum of Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Phyllium (Phyllium) jacobsoni Rehn & Rehn, 1933
| Hennemann, Frank H., Conle, Oskar V., Gottardo, Marco & Bresseel, Joachim 2009 |
Phyllium jacobsoni
| Grosser, D. 2008: 122 |
| Otte, D. & Brock, P. 2005: 274 |
| Zompro, O. & Grosser, D. 2003: 136 |
| Grosser, D. 2001: 84 |
| Klante, H. 1976: 70 |
Phyllium jacobsoni
| Rehn, J. A. G. & Rehn, J. W. H. 1933: 419 |
Phyllium bilobatum
| Klante, H. 1976: 71 |
| Redtenbacher, J. 1906: 177 |
Phyllium siccifolium , Redtenbacher, 1906: 176
| Redtenbacher, J. 1906: 176 |
Phyllium geryon , Griffini, 1898a: 11
| Caudell, A. N. 1927: 19 |
| Giglio-Tos, E. 1910: 16 |
| Griffini 1898: 11 |
| Griffini 1898: 2 |