Pheretima kitangladensis, James, 2004
|
publication ID |
https://doi.org/10.5281/zenodo.4618925 |
|
DOI |
https://doi.org/10.5281/zenodo.10528190 |
|
persistent identifier |
https://treatment.plazi.org/id/9918E954-FFB5-E063-09AD-FE595108FE31 |
|
treatment provided by |
Carolina |
|
scientific name |
Pheretima kitangladensis |
| status |
sp. nov. |
Pheretima kitangladensis , new species
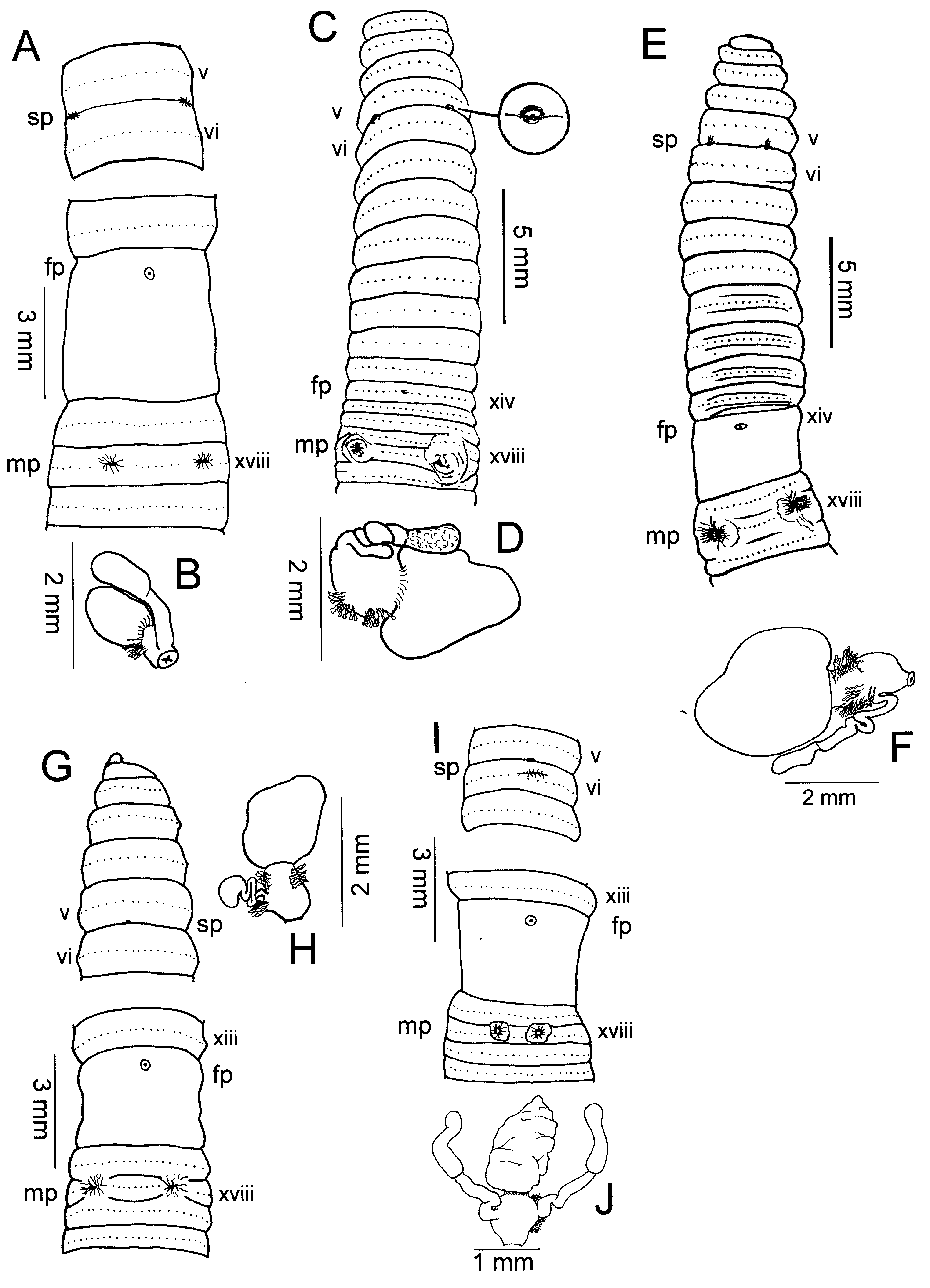
( Figs. 1A, B View Fig )
Material examined. – Holotype - adult ( NMA 003973 ) Philippines, Mindanao Island, Bukidnon Province, Mt. Kitanglad Range , 18.5 km S, 4 km. E of Camp Philips, 2250 m. elevation, coll. L. Heaney, 2 Apr.1993.
Etymology. – The species is named after the mountain range on which it was found.
Description. – Dorsal brown pigment, but pigment lacking in narrow band on segmental equators, unpigmented band sharply constricted at mid-dorsal line. 108 x 3.8 mm (x), 3.5 mm (clitellum), 3.6 mm (xxv), 99 segments; body cylindrical in cross-section, gradually tapered towards head and tail. First dorsal pore 12/13, spermathecal pores paired in 5/6, 0.28 circumference apart; female pore single on papilla in xiv, openings of copulatory bursae paired in xviii, 0.20 circumference apart in 7 th or 8 th setal line, 8 setae between openings. Setae regularly distributed around segmental equators, 44 setae on vii, 48 setae on xx; no dorsal or ventral gaps in setal spacing. Clitellum annular xiv-xvi; no genital markings ( Fig. 1A View Fig ).
Septa 4/5-6/7, 7/8 muscular, 8/9 thin, 9/10 lacking, 10/11- 12/13 muscular. Nephridia in dense tufts on anterior faces of 5/6, 6/7; nephridia of post-clitellar segments in pre-, postseptal rows near septum-body wall junction.
Large gizzard in viii, esophagus with vertical lamellae x-xiii, intestinal origin xvi, simple caeca originating in xxvii, extending forward to xxvi, ventral margins with 3-5 incisions; paired ventral ridges of intestine from mid-ventral in xix to openings of caeca; typhlosole simple fold 1/5 lumen diameter, xxvii-lxviii; 32 longitudinal vessels within intestinal wall xxvii-xl.
Hearts x-xiii esophageal, commissural vessels vi, vii, ix lateral; viii to gizzard; ventral vessel with branches to body wall in viii. Supra-esophageal vessel x-xiii, extra esophageal vessels to ventral esophageal wall in x, efferent parietoesophageal vessels not seen.
Ovaries and funnels free in xiii, spermathecae paired in vi with nephridia on spermathecal ducts; each spermatheca with large ampulla, single stalked diverticulum terminating in clubshaped receptacle ( Fig. 1B View Fig ). Male sexual system holandric, testes, funnels enclosed in paired sacs in x, xi; seminal vesicles xi, xii with dorsal lobes; vasa deferentia free from body wall en route to ental end of prostatic ducts; each prostate racemose occupying xvi-xviii, with stout muscular duct entering central of three bulges of the copulatory bursae in xviii; coelomic surface of copulatory bursae lacking glandular or other projections; anterior and posterior bulges house thick muscular pads flanking transverse ridge occupying place of penis on roof of copulatory bursa.
Remarks. – Pheretima kitangladensis keys to the urceolata group in Sims & Easton (1972), which is composed of two species, P. urceolata ( Horst, 1893) and P. baweanensis ( Michaelsen, 1924) . Gates (1961) synonymized these two species and P. ditheca ( Michaelsen, 1928) . These are from Sumatra, Bawean Island, and Borneo, respectively, and have lengths of 70-80mm, 170mm, and 44mm, respectively. Gates (1961) also assigned a Philippine specimen to P. urceolata . The collection location was given as “Mt. Apo, east slope at 6200 feet, Davao Province, Mindanao.” Gates suspected human intervention helped to attain this extensive distribution. He admitted that the size range encompassed by adult P. urceolata s.l. ( 44 mm to 170 mm) seemed “unusual.” However, Sims & Easton (1972) placed P. ditheca in Metaphire , because nephridia were not recorded from the spermathecal ducts, a character reported for other species in Michaelsen (1928). Thus the low end of the size range could be restated as about 70 mm. Human intervention is one hypothesis for the range of P. urceolata , but resolution will require more material from Indonesia.
If P. ditheca turns out to have nephridia on the spermathecal ducts, some information suggests its inclusion in the synonymy of P. urceolata may be premature. Michaelsen (1928) gave the size of P. ditheca as that of the largest individual in the lot. He stated the separation of the male pores as about 0.25 of the circumference, and the spermathecal pores at 0.43, which are much less and much greater than the respective pore separations in the type material of P. urceolata (see Table 1). He notes the absence of the septa 8/9/10 and the presence of “Chylustaschenstruktur” in the esophageal wall of segments x-xiii, as well as the sharp narrowing of the spermathecal duct as it enters the body wall ( Michaelsen, 1928: p. 47, Fig. 12). This last is in contrast to the relatively thick ducts “slightly narrowed at parietes” given in Gates’ definition of P. urceolata (1961: p 298).
The presence of a single pair of spermathecae opening on 5/ 6 defines the species group sensu Sims & Easton (1972) or the species according to Gates. Assuming all for now P. urceolata are conspecific, the species described here differ from it and from one another sufficiently to merit species rank. Table 1 contains information on the characters used to distinguish among the members of the urceolata group. Pheretima kitangladensis differs from P. urceolata in having more setae per segment, no dorsal setal gap, more closely spaced spermathecal pores and more widely spaced male pores. Internally, P. kitangladensis lacks the thick septum 9/10 reported for P. urceolata in Sims and Easton (1972), has two well-developed muscular pads within each copulatory pouch and a thin ridge in place of each penis seen in P. urceolata . All information on P. urceolata makes no mention of the presence of vertical lamellae in the esophagus, except for the questionable P. ditheca , which may be a Metaphire .
The locality of P. kitangladensis is quite remote from human disturbance and activity of the sort likely to cause introduction of earthworms from Indonesia, or anywhere else. In all the collections on which this paper is based, only the lowest elevation site ( 1100 m.) yielded a peregrine species, the glossoscolecid Pontoscolex corethrurus (Müller, 1857) . It is highly unlikely that P. kitangladensis is an exotic.
The following worm in the Natural History Museum, London, was labeled as P. urceolata :
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.