Perinereis anderssoni Kinberg, 1865
|
publication ID |
https://doi.org/ 10.5852/ejt.2021.787.1619 |
|
publication LSID |
lsid:zoobank.org:pub:6E595BC0-37AB-460E-B0EB-435576CDD207 |
|
DOI |
https://doi.org/10.5281/zenodo.5841095 |
|
persistent identifier |
https://treatment.plazi.org/id/03948791-CB20-2921-FD8D-FBBE9B79D1AC |
|
treatment provided by |
Felipe |
|
scientific name |
Perinereis anderssoni Kinberg, 1865 |
| status |
|
Perinereis anderssoni Kinberg, 1865 View in CoL
Perinereis anderssoni Kinberg, 1865: 175 View in CoL .
Nereis minor Hansen, 1882: 12 View in CoL , pl. 4 figs 8 – 12.
Perinereis anderssoni View in CoL – Hartman 1948: 72–73. — De León-González & Solís-Weiss 1998: 675, figs 1a – g (partim).
Material examined
BRAZIL • 7 specs; Rio de Janeiro, Praia Vermelha ; 16 Aug. 2012; M. Coutihno and V. Schawn leg.; ECOSUR P3208 View Materials .
Description
BODY AND MEASUREMENTS. Non-type specimens (ECOSUR P3208) in good conditions, complete, largest specimen 23 mm long, 1.9 mm wide at chaetiger 10 excluding parapodia, 67 chaetigers. Body dorsally pigmented, with reddish brown pigmentation in palps, prostomium and chaetigers, intense in anterior region, progressively discoloring posteriorly ( Fig. 2A–B View Fig ). Pale lines present in dorsum of first 12 chaetigers ( Fig. 2A View Fig ), fingerprint-like pattern from chaetiger 13.
PROSTOMIUM. Subpyriform, as long as wide; anterior region distally entire, rectangular, slightly longer than posterior region, dorsal groove present ( Fig. 2A View Fig ); anterolateral gap between antenna and palpophore as long as diameter of antennae.
ANTENNAE. Digitiform, not passing palps, lacking pigments, half as long as prostomium, gap between them as long as basal wide of antennae ( Fig. 2A View Fig ).
PALPS. Palpophores ovoid, swollen, 1.5 × as long as wide, shorter than prostomium, subdistal transverse groove present ( Fig. 2A View Fig ). Palpostyles rounded.
EYES. Rounded, anterior and posterior pairs subequal, in trapezoidal arrangement, posterior pair covered by anterior margin of tentacular belt ( Fig. 2A View Fig ).
TENTACULAR BELT. 1.5× as long as chaetiger 1, covering posterior pair of eyes, anterior dorsal margin straight ( Fig. 2A View Fig ).
TENTACULAR CIRRI. Moniliform, not jointed, basal segment largest, remaining ones decrease in size progressively toward distal end longest cirri reaching end of chaetiger 1 ( Fig. 2A View Fig ).
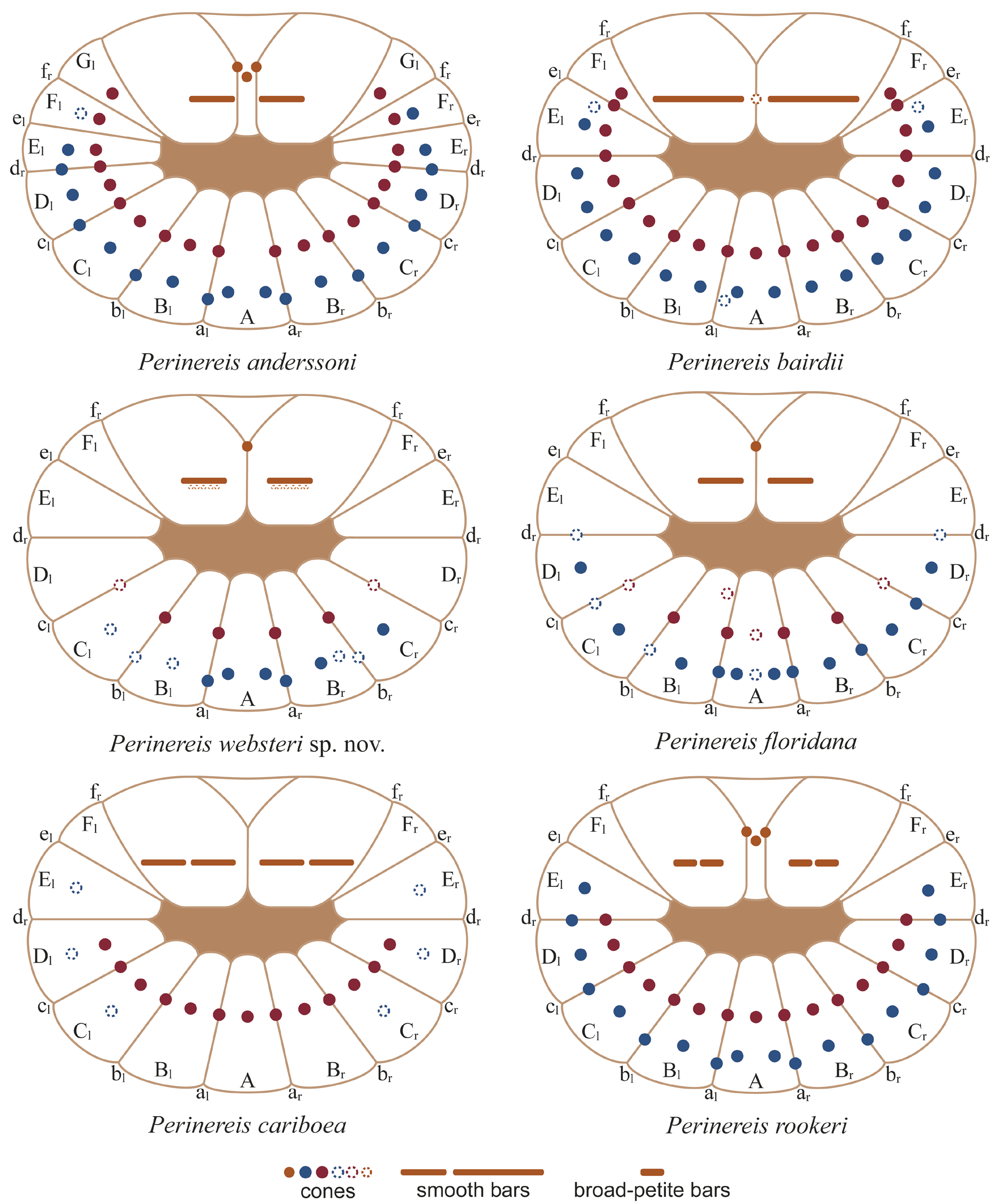
PHARYNX. Dissected; jaws brown, cutting edge with 10 rounded teeth ( Fig. 2D View Fig ). Maxillary ring: I = 5 cones in triangle; II = 11–12 cones in arc; III = 19 cones in rectangle; IV = 19–33 cones in arc. Oral ring: V = 3 cones in triangle, two cones over each lateral edges; VI = 1–1 smooth bars; VII–VIII = 40 cones in two bands: anterior band with 8 paragnaths in a furrow row with 1 cone on the regions a–d, and 12 paragnaths in ridge row with 1 cone on each B–G regions; posterior band with 8 paragnaths in furrow row with 1 cone on the regions a–d, and 12 paragnaths in ridge row with 2 cones on region A and 1 cone on the regions B–F ( Fig. 13 View Fig ). Furrow pattern of areas VI–V–VI, Π- shaped ( Fig. 13 View Fig ).
DORSAL CIRRI. Digitiform in first chaetigers, filiform thereafter, attached basally to dorsal ligule in anteriormost chaetigers, medially in middle chaetigers, and subdistally in posterior chaetigers ( Fig. 2E– I View Fig ); 1.5 × as long as distal lobe of dorsal ligule in chaetiger 1, 2.2 × in chaetiger 10, 2.3× in chaetiger 28, 2.5× in chaetiger 45, 3.2× in chaetiger 62 ( Fig. 2E–I View Fig ); 4.7 × as long as proximal lobe of dorsal ligule in chaetiger 1, 2.7 × in chaetiger 10, 2.6 × in chaetiger 28, 1.5 × in chaetiger 45, 1× length in chaetiger 62 ( Fig. 2E–I View Fig ).
DORSAL LIGULES. Subconical with blunt tip in anterior and middle chaetigers, becoming pennant-like toward posterior chaetigers, with distal lobes longer than proximal ones in first chaetigers, becoming as long as in anterior and shorter than in middle and posterior chaetigers ( Fig. 2E–I View Fig ). Distal lobe of dorsal ligule subconical throughout; as long as median ligule in chaetigers 10 and 28, 1.8 × as long as in chaetiger 45, 2.8× in chaetiger 62 ( Fig. 2E–I View Fig ).
MEDIAN LIGULES. Subconical with blunt tip in anterior chaetigers, becoming sharper thereafter ( Fig. 2E– I View Fig ); 1 × length of neuroacicular ligule in chaetiger 10, 4.3× in chaetiger 28, 2× in chaetigers 45 and 62 ( Fig. 2E–I View Fig ).
NEUROACICULAR LIGULES. Subconical in anterior and middle chaetigers, becoming rounded in posterior ones ( Fig. 2E–I View Fig ); 0.7× length of ventral ligule in chaetiger 1, 2× in chaetiger 10, 1 × in chaetiger 28, 2× in 45, 1.6 × in chaetiger 62 ( Fig. 2E–I View Fig ).
NEUROPODIAL SUPERIOR AND INFERIOR LOBES. Present in anterior and middle chaetigers, both rounded, inferior one wider than superior one throughout ( Fig. 2E–I View Fig ).
NEUROPODIAL POSTCHAETAL LOBES. Rounded, half as long as neuroacicular ligule throughout.
VENTRAL LIGULES. Digitiform throughout ( Fig. 2E–I View Fig ).
VENTRAL CIRRI. Digitiform throughout ( Fig. 2E–I View Fig ); 1× length of ventral ligule in chaetiger 1, 0.8× length thereafter ( Fig. 2E–I View Fig ).
ACICULAE. Dark brown throughout ( Fig. 2A–I View Fig ); notoaciculae absent in first two chaetigers ( Fig. 2E View Fig ). NOTOCHAETAE. All homogomph symmetrical spinigers. Blades of spinigers with basal pectinate, coarse teeth, becoming minute toward distal end.
NEUROCHAETAE. Homogomph symmetrical spinigers and heterogomph falcigers in supra-acicular fascicles, heterogomph spinigers and falcigers in sub-acicular fascicles. Neuropodial homogomph spiniger as notopodial ones. Heterogomph spinigers with blades pectinate, minute teeth and decreasing in size toward distal end. Heterogomph falcigers pectinate, long and narrow teeth, half of inner edge of blade dentate, distal tips stout, stouter in supra-acicular falcigers ( Fig. 2J–K View Fig ).
PYGIDIUM. Crenulated, bilobate ( Fig. 2B View Fig ); anal cirri subulate, as long as last three chaetigers ( Fig. 2B View Fig ).
Remarks
Kinberg (1865) included few features in the original description of P. anderssoni , all focused on features of the anterior end of the body. Hartman (1948) briefly redescribed the species and regarded Nereis bairdii Webster, 1884 , and doubtfully P. ponteni Kinberg, 1865 , as junior synonyms. Morphological differences between P. anderssoni and P. ponteni have been pointed out in recent studies (e.g., Coutinho & Santos 2014; Coutinho et al. 2015), and here the synonymy of N. bairdii is rejected (see remarks of Perinereis bairdii below). Epitokes were not available for this study, but recently Peixoto & Santos (2016) detailed the reproductive biology of P. anderssoni , finding four epitokal stages in both males and females.
De León-González & Solís-Weiss (1998) redescribed P. anderssoni based on the best-preserved specimen of the syntype series, but they highlighted the poor conditions of the type series and the absence of pigmentation. To improve the available redescription, a complementary description is provided based on topotypes in better condition of preservation. De León-González & Solís-Weiss (1998) also recorded the species from Chile and the Gulf of Mexico, regarding it as amphiamerican. The inclusion of material from Juan Fernández, Chile is explained after Hartman (1948) noted some similarities in parapodia between P. anderssoni and P. pseudocamiguina ( Augener, 1922) (type locality: Juan Fernández) ( Augener 1922; Hutchings et al. 1991), but there are differences between them: 1) P. pseudocamiguina has 1–2 cones in area I, 7–13 cones in area III, and occasionally bars in areas IV; whereas P. anderssoni has 4–5 cones in area I, 19–21 cones in areas III, and no bars in areas IV; 2) in P. pseudocamiguina , dorsal cirri in posterior chaetigersare 0.5–0.6×as long as proximal lobe of dorsal ligule, whereas in P. anderssoni they ate as long as; 3) in P. pseudocamiguina , dorsal cirri are 2.0–2.2 × as long as distal lobe of dorsal ligules, whereas in P. anderssoni they are 3.2 × longer; 4) in P. pseudocamiguina , the dorsal ligules in posterior chaetigers are longer than wide and with dorsal surface slightly convex; whereas in P. anderssoni , they are wider than long and with dorsal surface strongly convex. Finally, Hartman (1948), following Augener (1934), also considered N. minor Hansen, 1882 (type locality: Rio de Janeiro, Brazil) as a probable junior synonym of P. anderssoni ; the original description is succinct, but the illustrations included show the high similarities in the arrangement of paragnaths and the parapodia (probably from anterior chaetigers), so the synonymy with N. minor is retained until a further revision of type material.
Liñero-Arana & Reyes-Vásquez (1979) reported P. anderssoni from Venezuela, but based on the redescription and the current description, there are key differences between Venezuelan specimens and P. anderssoni : 1) in Venezuelan specimens, numbers of paragnaths in some pharyngeal areas as follows: I = 10–18, II = 20–27, V = 1, whereas in P. anderssoni are as follows: I = 5, II = 11–12, V = 3; 2) in Venezuelan specimens, dorsal cirri in posterior chaetigers are 0.6× as long as proximal lobes and 1.5× as long as distal lobes of dorsal ligules, whereas in P. anderssoni , dorsal cirri are as long as proximal lobes and 3.2× as long as distal lobes of dorsal ligules; 3) in Venezuelan specimens, the dorsal ligules are at least 3.5× as long as median ligules and the ventral ligules are 2–4 × longer than neuroacicular ligules in posterior chaetigers, whereas in P. anderssoni , the dorsal ligules are 2.8 × as long as median ligules and ventral ligules are shorter than neuroacicular ligules. These specimens are closer to P. ponteni or P. bairdii than to P. anderssoni because of the large number of paragnaths in area I and a single paragnath in area V, recorded a couple of times ( Díaz-Díaz & Liñero-Arana 2002; Vanegas-Espinosa et al. 2007). The two Perinereis species described from Venezuela, P. mochimaensis Liñero-Arana, 1983 and P. cariacoensis Liñero-Arana, 1983 , are also different, therefore Venezuelan specimens belong to another, undescribed species.
Following Hartman (1948), Rioja (1960) reported P. anderssoni for Veracruz, Mexico but recognizing that the specimens are closer to P. ponteni . Key differences among these Mexican specimens and P. anderssoni are the following: 1) in Mexican specimens, area I has 10–12 cones and area V has 1 cone, whereas in P. anderssoni , area I has 5 cones and area V has 3 cones; 2) in Mexican specimens, antennae are 0.3 ×as long as prostomium, whereas in P. anderssoni they are half as long; 3) in Mexican specimens, dorsal cirri in anterior chaetigers are shorter than distal lobes of dorsal ligules, whereas in P. anderssoni they are longer; 4) in Mexican specimens, dorsal cirri are 2× as long as distal lobes of dorsal ligules and ventral ligules are 2.5 × as long as neuroacicular ligules in posterior chaetigers, whereas in P. anderssoni dorsal cirri are 3.2 × longer and the ventral ligules are shorter than. Salazar-Vallejo & Jiménez-Cueto (1997) reported P. anderssoni for the Mexican Caribbean, but these specimens differ from Brazilian ones in the following features: 1) in the Caribbean specimens, dorsal cirri are 1.4× as long as distal lobes of dorsal ligules and median ligules are as long as neuroacicular ligules in anterior chaetigers, whereas in the Brazilian ones the dorsal cirri are 2.2 × longer and median ligules are subequal; 2) in the Caribbean specimens, dorsal cirri are 0.5× as long as proximal lobes and as long as distal lobes of dorsal ligules in posterior chaetigers, whereas in the Brazilian ones the dorsal cirri are as long as proximal lobes and 3.2× as long as distal lobes. Records of P. anderssoni in other Caribbean regions (e.g., Fauchald 1977; Ibárzabal 1986) deserve a new evaluation.
Distribution
Brazil.
| ECOSUR |
El Colegio de la Frontera Sur (Mexico) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Perinereis anderssoni Kinberg, 1865
| Conde-Vela, Víctor Manuel 2022 |
Perinereis anderssoni
| de Leon-Gonzalez J. A. & Solis-Weiss V. 1998: 675 |
| Hartman O. 1948: 72 |
Nereis minor
| Hansen G. A. 1882: 12 |
Perinereis anderssoni
| Kinberg J. G. H. 1865: 175 |