Oxychilus (Drouetia) viridescens, De Frias Martins, António M., Brito, Carlos P. & Backeljau, Thierry, 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3619.3.5 |
|
publication LSID |
lsid:zoobank.org:pub:4FB7A97C-D25F-41C8-8C62-2664E6E6F148 |
|
DOI |
https://doi.org/10.5281/zenodo.5619901 |
|
persistent identifier |
https://treatment.plazi.org/id/915F87CB-1228-FFED-519A-1635FD41C1FB |
|
treatment provided by |
Plazi |
|
scientific name |
Oxychilus (Drouetia) viridescens |
| status |
sp. nov. |
Oxychilus (Drouetia) viridescens View in CoL n.sp.
Helix atlantica Morelet & Drouët, 1857: 149 (partim); Morelet, 1860: 167 (partim).
Types. Holotype ( Figs. 2 View FIGURE 2 A, 4A, C; Table 2 View TABLE 2 , Ov1): Natural History Museum, London (NHMUK 20100658). Paratypes: Natural History Museum, London (2 specimens NHMUK 20100659); Muséum National d’Histoire Naturelle, Paris (1 specimen MNHN 24265); National Museum of Natural History, Washington (2 specimens USNM 1155712 and 1155713); Museum of Comparative Zoology, Harvard University, Cambridge (1 specimen MCZ 373862); Royal Belgian Institute of Natural Sciences, Brussels (1 specimen IG 31765 (MT 2329); Museum of Natural History, Wroclaw University, Poland (1 specimen MP 1011); Department of Biology, University of the Azores, Portugal (7 specimens DB/UAç-MT 1423 and 1424).
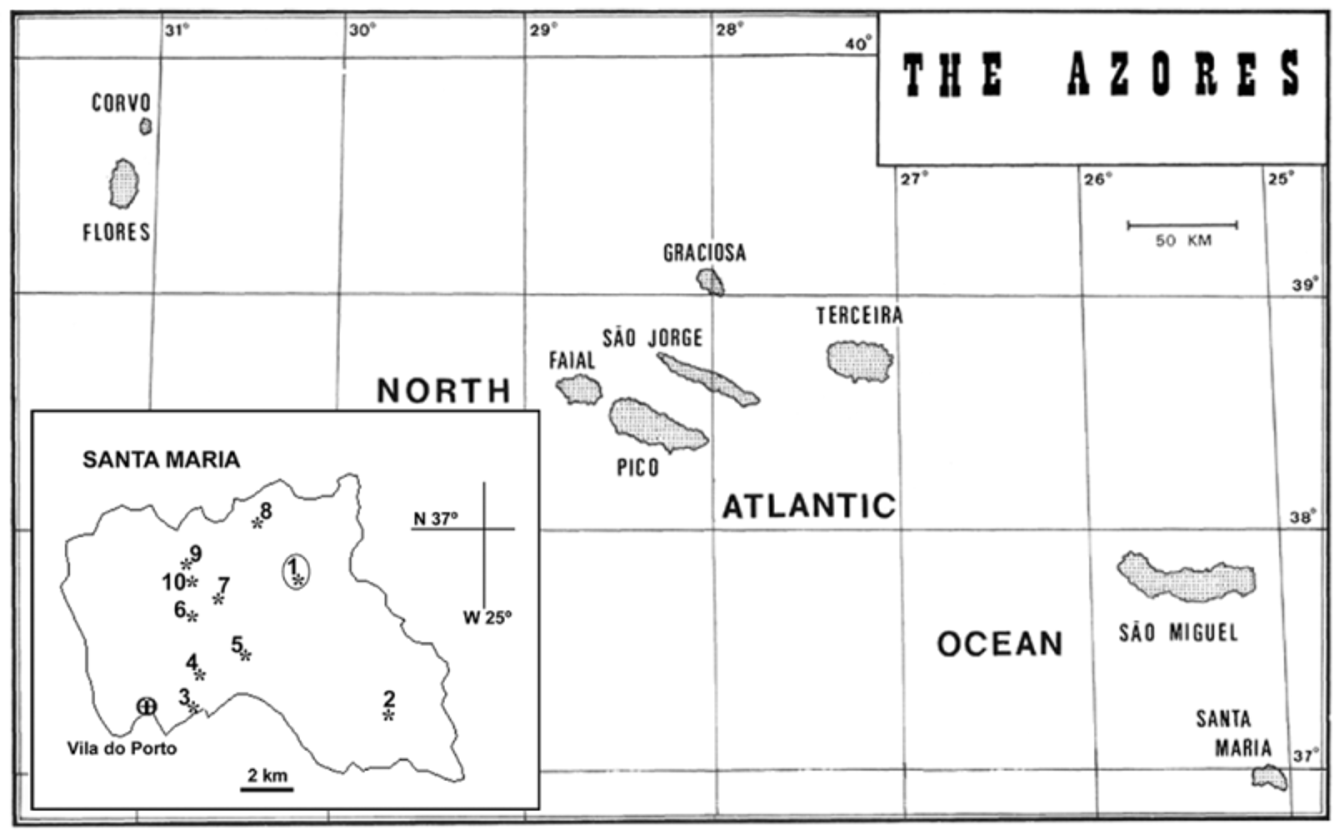
Type locality. Ribeira Funda, Feteiras de Santa Bárbara, Santa Maria, Açores (N 36º 59’ 57”, W 25º 05’ 03”) ( Figure 1 View FIGURE 1 and Table 1 View TABLE 1 , Sta 1)
Etymology. The name refers to the characteristic greenish color of the shell.
Diagnosis. Shell diameter 8 mm, greenish, umbilical region grayish, columellar lip and parietal lip almost at the same plane; penis very thin, penial caecum long; epiphallus with two distinct swellings, proximal one attached to penial sheath, distal one attached to penial constriction; distal vagina glandular.
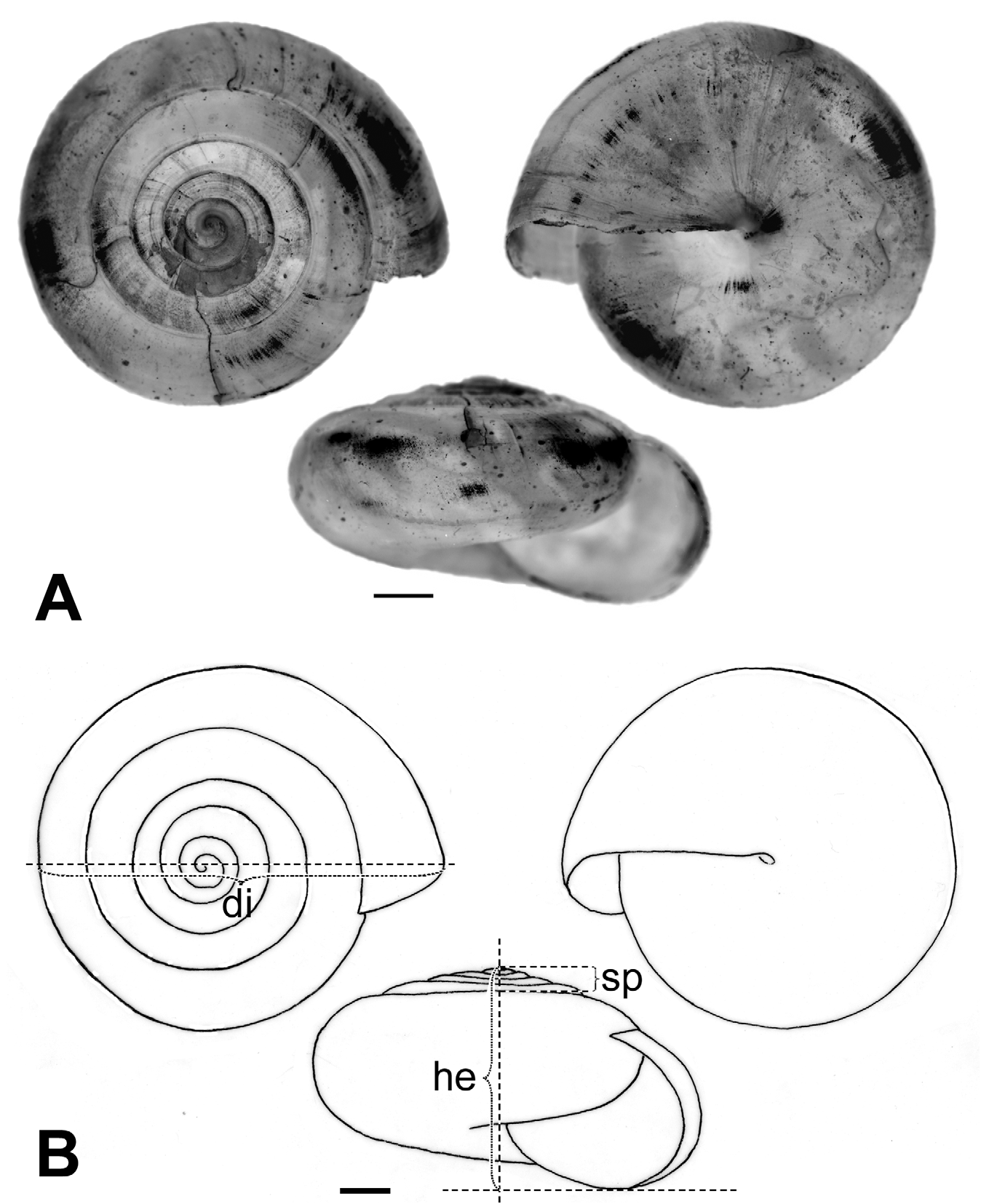
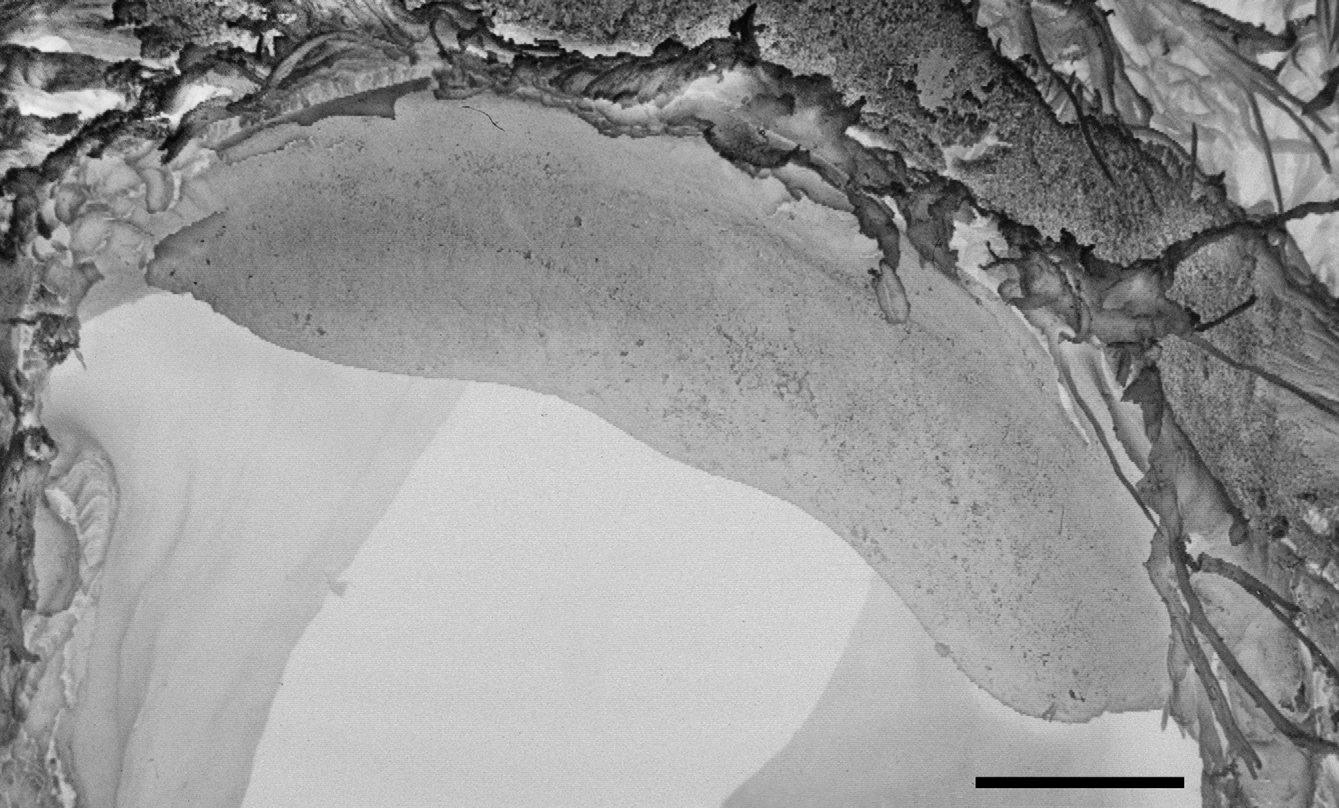
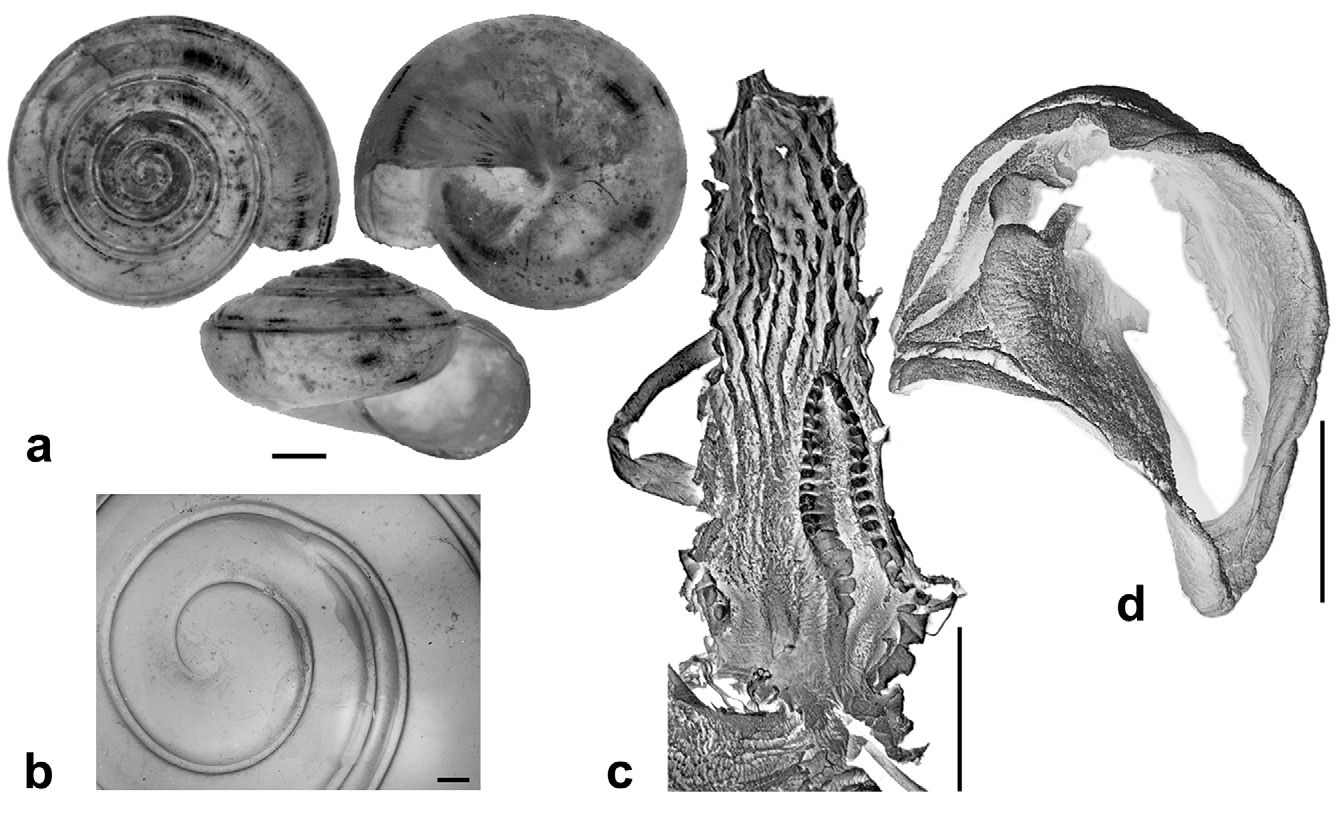
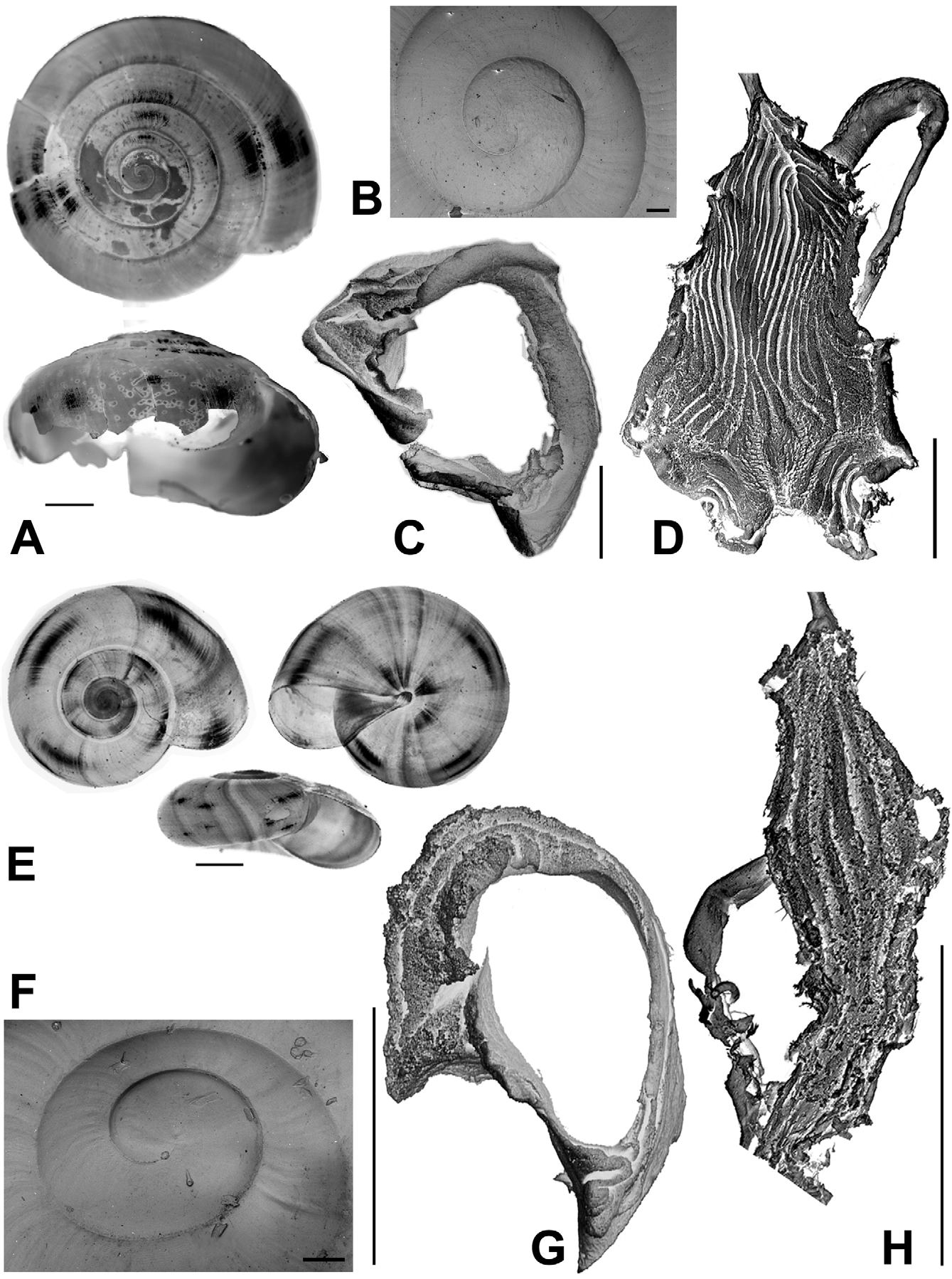
Description. Shell ( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 , 4 View FIGURE 4 C; Table 2 View TABLE 2 ) up to 8.2 mm in diameter and 4.8 mm in height, non-umbilicate, somewhat solid, translucent, glossy, conspicuously golden green, with very fine, microscopic spiral striae crossing dense and regular growth lines; spire low, with up to 6.2 flattened whorls, last whorl with a squarish profile. Aperture oblique, the columellar lip running almost at the same plane as the parietal lip (see Fig. 4 View FIGURE 4 C); outer lip sharp, columellar lip slightly reflected over the umbilical region which is grayish and covered by a tenuous, whitish callus. Protoconch not well defined, with very faint, fine spiral striae continuing more markedly on the teleoconch.
Animal ( Fig. 4 View FIGURE 4 ): neck blue with purplish hues changing gradually to light orange toward the foot, the darkly pigmented posterior tentacle retractors conspicuous through the dorsal neck skin; four dorsal white grooves run forward from the border of the mantle on the dorsal neck, the central ones to the posterior tentacles and front, the two lateral ones descending obliquely, on each side, to underneath the anterior tentacles; head and front with dark blue longitudinal streaks on a pinkish background; posterior tentacles long, dark grayish blue, clearer near base, anterior tentacles faintly blue with sparse brownish dots near the tips; posterior dorsal tip of foot dark blue; sole of the foot longitudinally tripartite, whitish green to dark yellow.
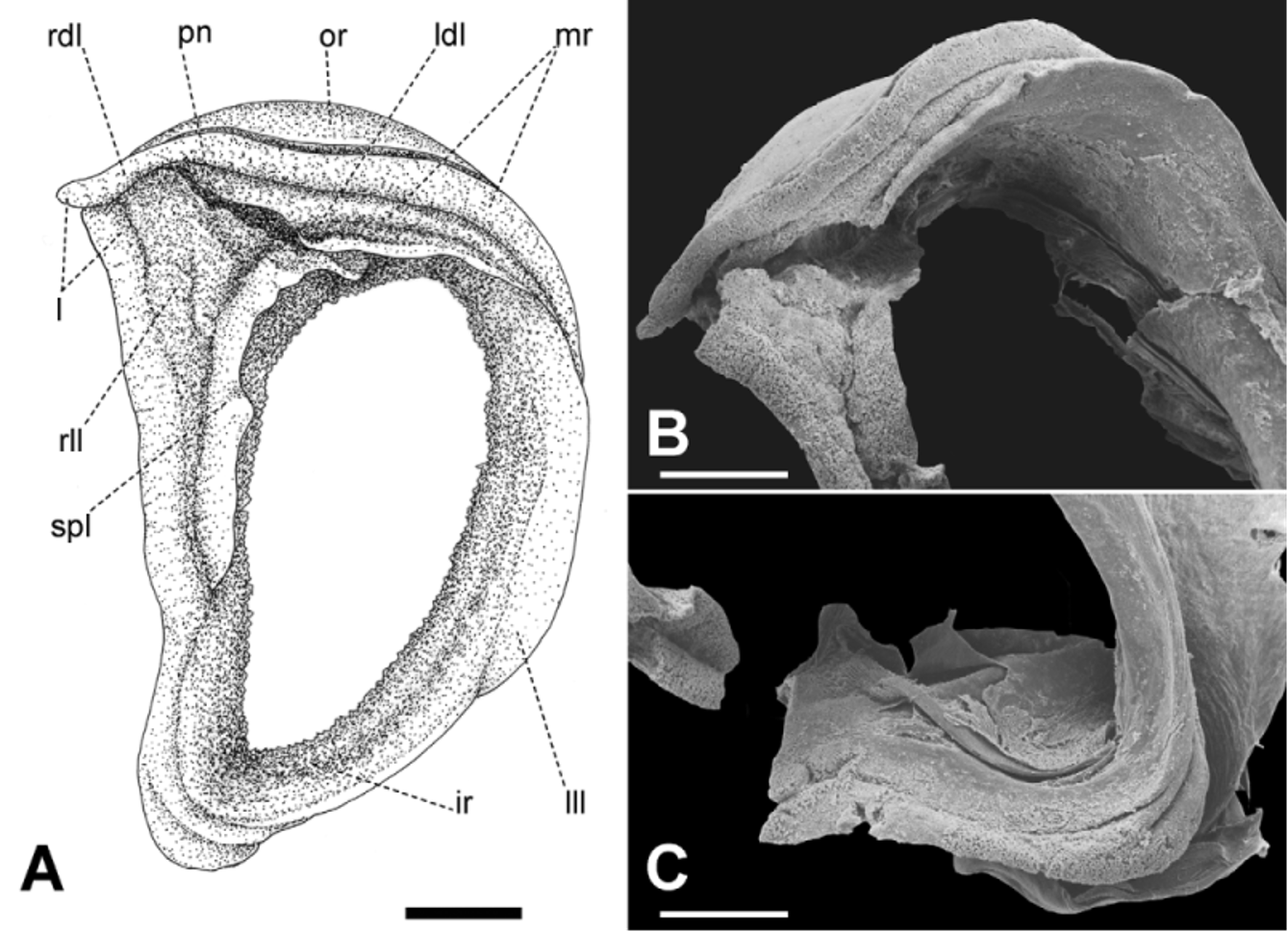
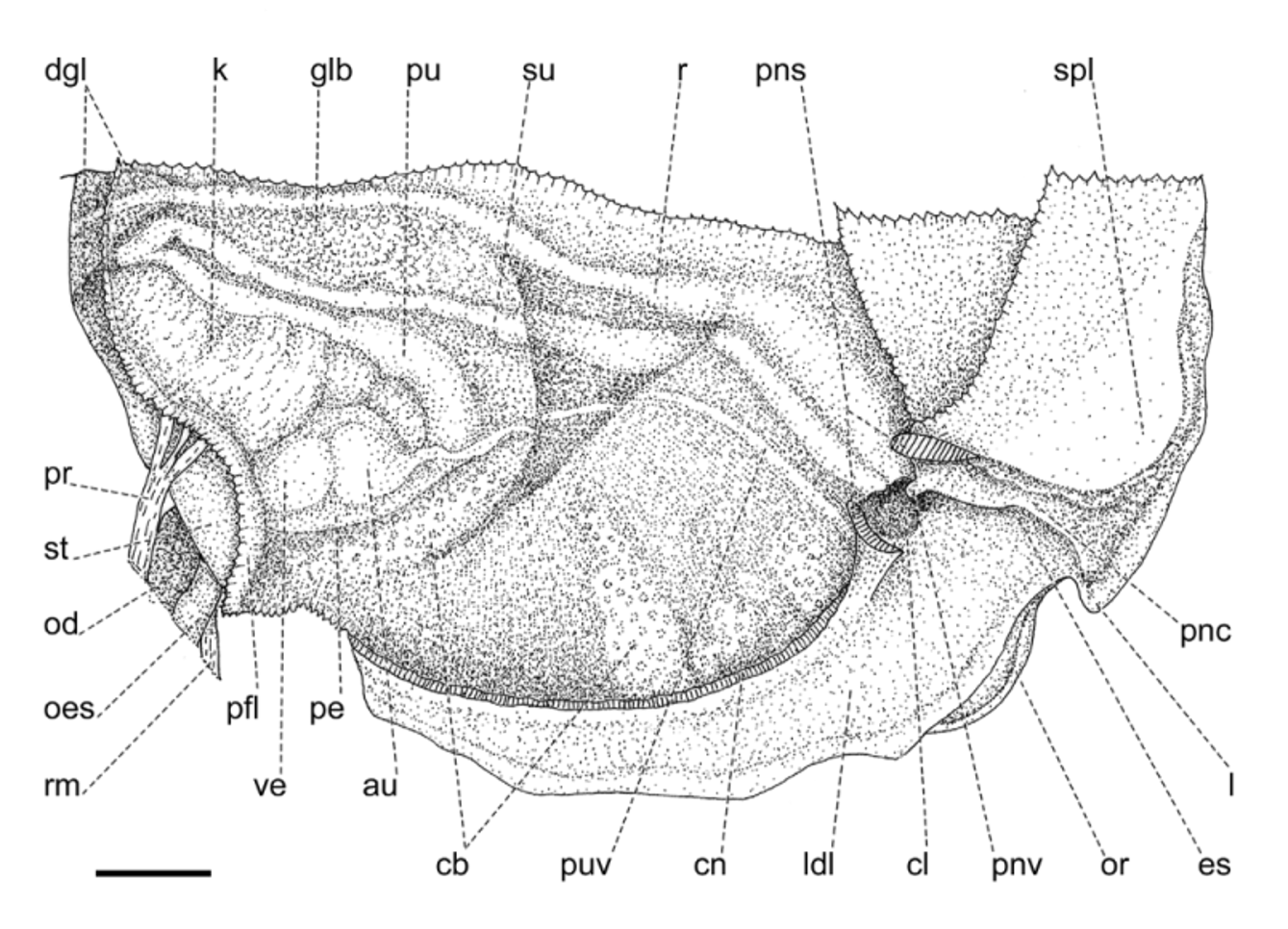
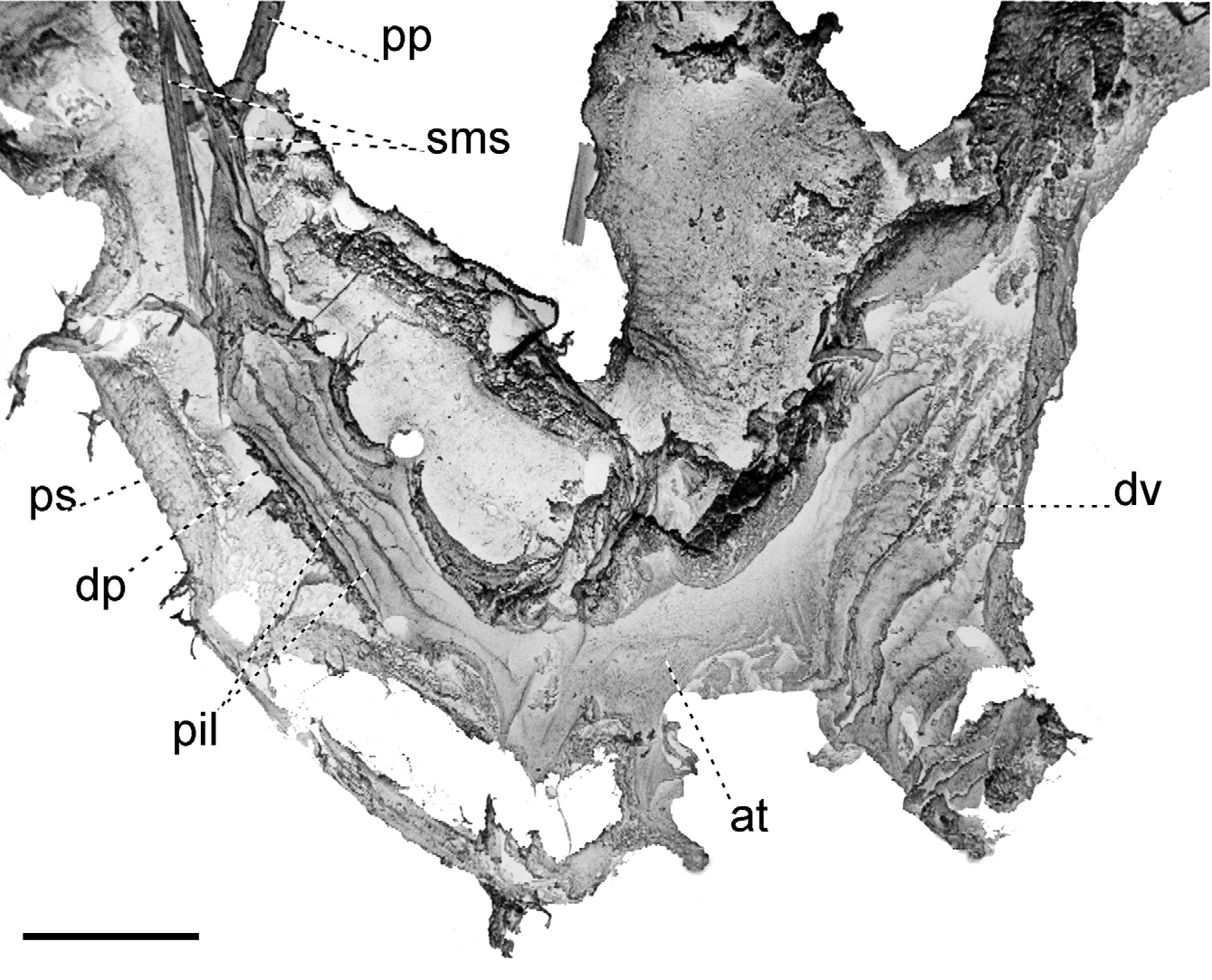
Mantle collar ( Figs. 4 View FIGURE 4 , 5 View FIGURE 5 ) dark blue, darker around the pneumostome and lappet, becoming lighter toward the left side of the animal. The three basic lobes of the mantle border (secretory, sensory and muscular) are present and are here referred to as rings, the term “lobe” being reserved to follow the current terminology for the description of this structure in helicids (e.g., Martins 2002). Outer, secretory ring, responsible for the secretion of the shell, thin, somewhat retracted behind the next ring, surrounding the entire border of the mantle. The middle, thick, originally sensory ring is bilobed and appears to be primarily of a glandular nature as indicated by its spongy appearance, and it is separated on its right side by the deep pneumostomal slit; right lateral lobe triangular, gently narrowing posteriorly from the lappet; right dorsal lobe forming the dorsal tip of the lappet and extending continuously outside the innermost, left dorsal lobe. Inner, muscular ring expanding to each side of the pneumostome in two wide and thin flaps, the subpneumostomal lobe and the left lateral lobe. The three rings compress posteriorly into a narrow, rounded canal.
The blotched coloration of the visceral mass is seen through the translucent, greenish shell; conspicuous dark spot just behind the pneumostomal area, the remaining few spots, of various sizes, yellowish, sparsely distributed over a brownish green to golden green background.
Pallial cavity ( Fig. 6 View FIGURE 6 ) elongated, deep; kidney bilobed, completely located in the pallial cavity, roughly triangular, with narrow and elongate anterior lobe squeezed between the heart and the primary ureter; sigmurethrous, secondary ureter bordering posterior lobe of kidney, then bending anteriorly as it approaches the rectum and following forward to open in a cloacal atrium, side-by-side with the rectum, near the pneumostome; a valve-like papilla, the pneumostomal valve, isolates the cloacal atrium from the pneumostomal aperture, thus separating the excretory slit from the pneumostomal canal. A thick, glandular body lies between the secondary ureter and the rectum, to the right of the kidney. Various white, possibly calcareous bodies aggregate in blotches irregularly dispersed throughout the roof of the pallial cavity.
Mandible ( Fig. 7 View FIGURE 7 ) oxygnathous, moderately long, strong, smooth, half-moon shaped, with convex median prominence on its free edge.
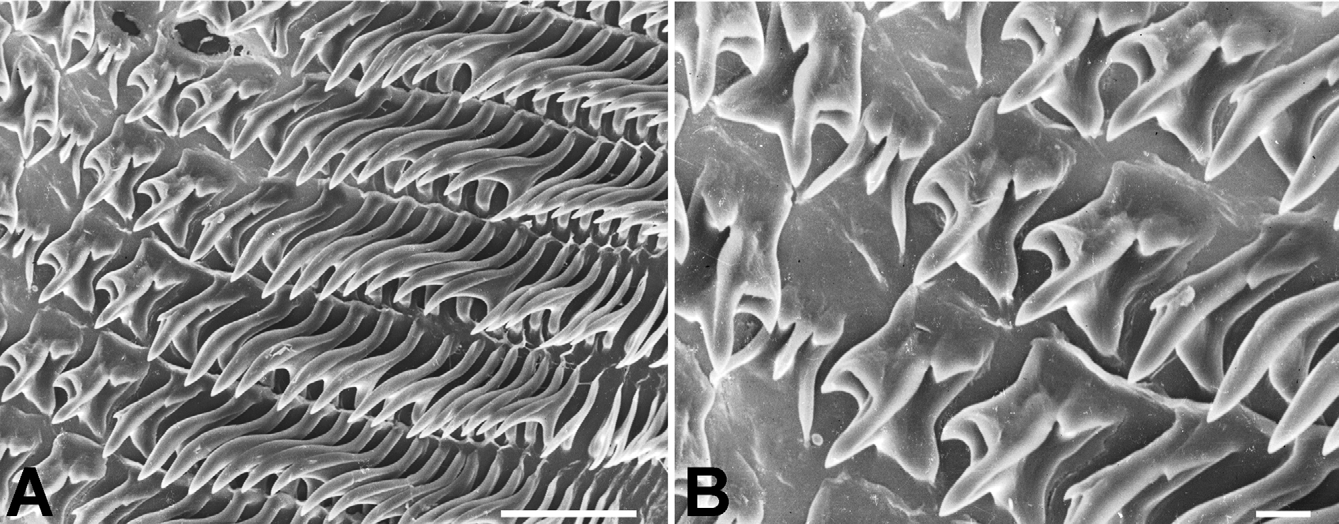
Radula ( Fig. 8 View FIGURE 8 ) [21+(1+2)+1+(2+1)+21]×50. Central tooth shorter and narrower than laterals, tricuspid; mesocone long, about half the length of the tooth, narrow, sharply pointed; ectocones small, sharp; base narrowing towards the crown, base line receding medially until at about the tip of the mesocone. First lateral tooth about twice as long as the central, tricuspid, endocone moderately long and merging medially into a basal tooth, receding laterally along with the base, with which it forms a pointed, long mesocone, the tip of which extends up to the arms of the base of the central tooth, ectocone short, triangular, wide, located far back in the crown; second lateral tooth similar to the first, but larger; a third lateral tooth of the transitional (intermediate) type is also tricuspid but much narrower than the other two, the shape resembling a marginal tooth, with both endocone and ectocone very small, sharp, mesocone long, curved and pointed. Marginal teeth falciform, retaining only the mesocone which is long, curved and pointed; first marginal teeth about the size of the transitional tooth, decreasing in size towards the outer edge of the row.
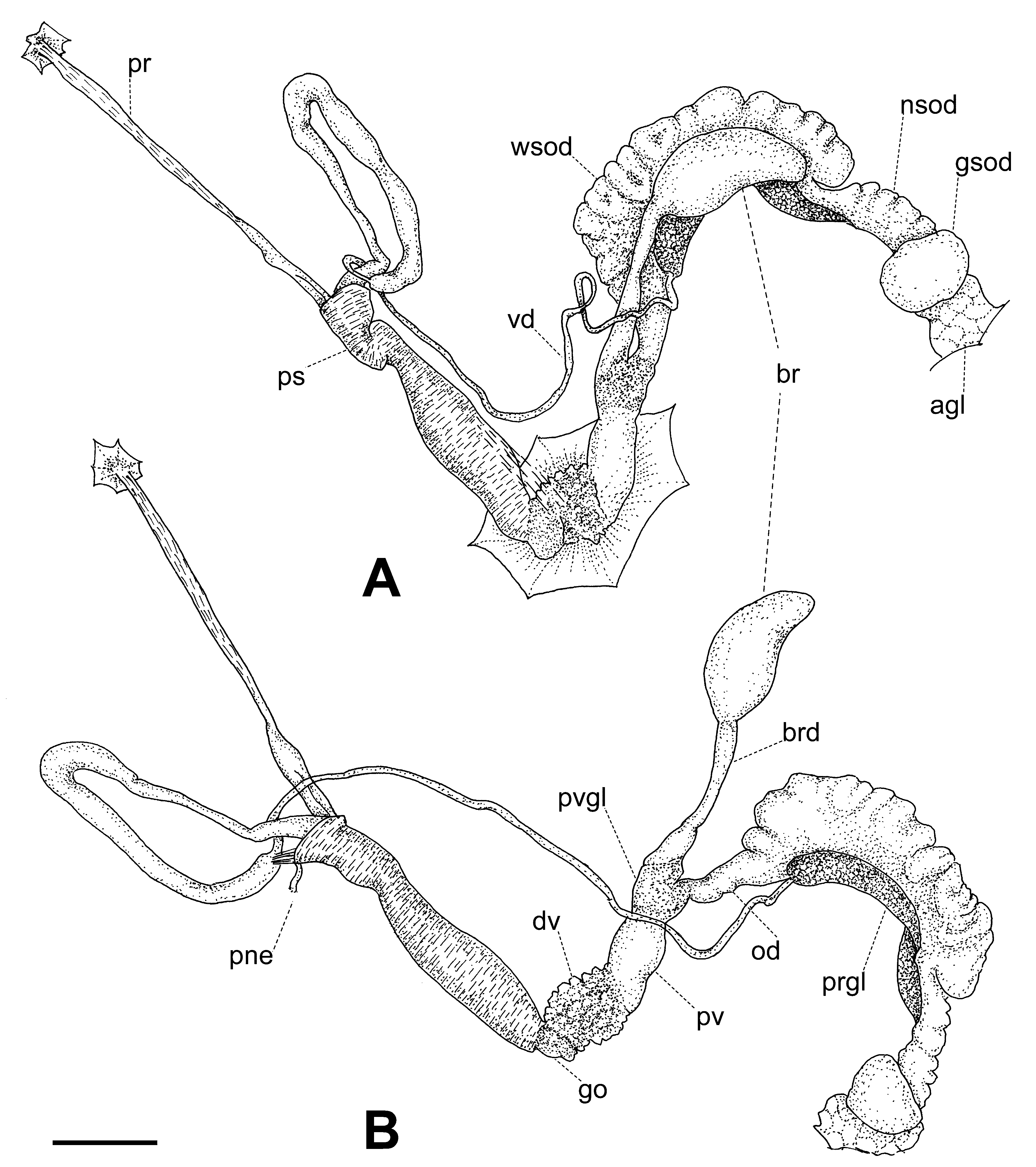
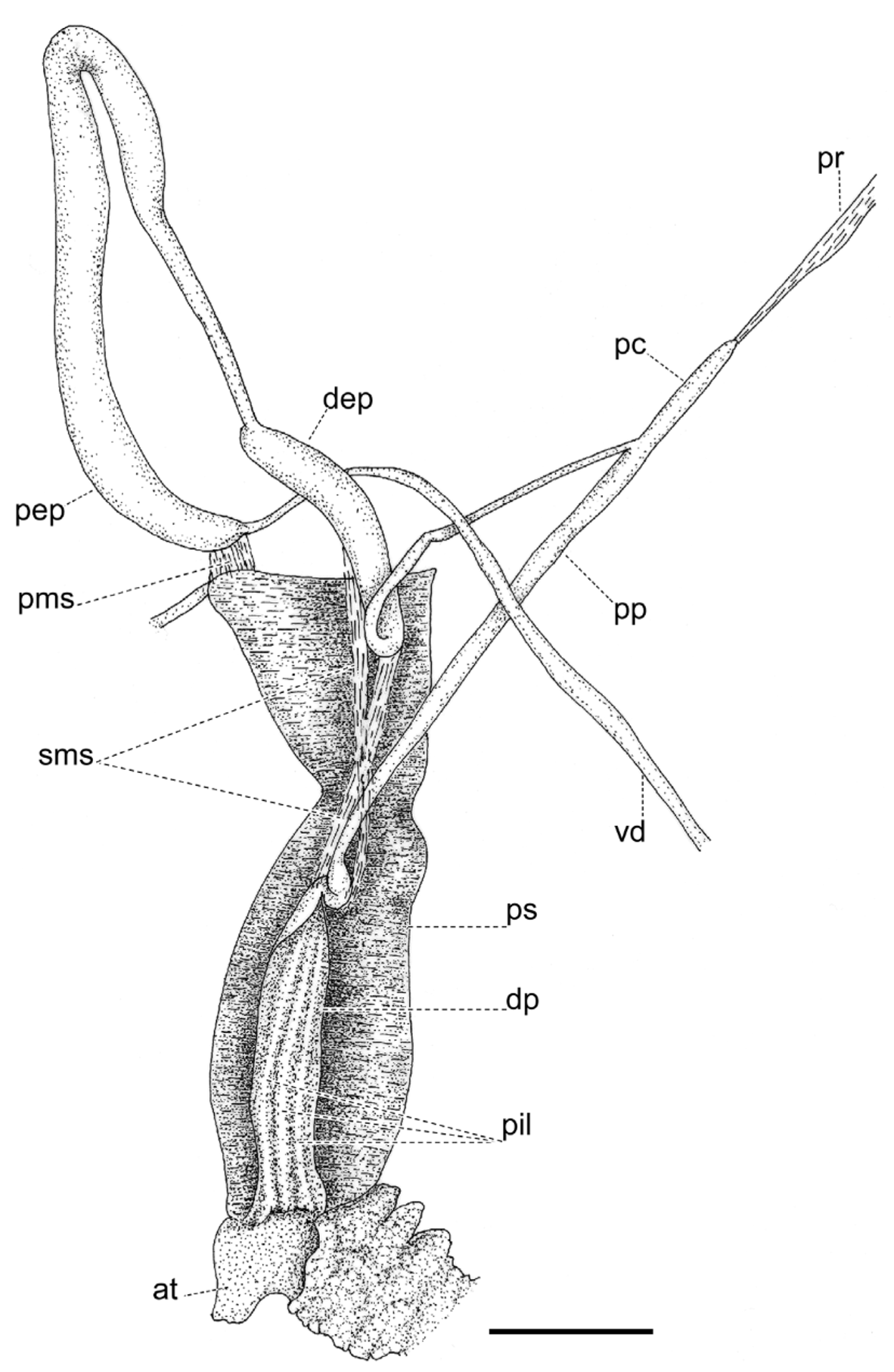
Reproductive system ( Figs. 9–11 View FIGURE 9 View FIGURE 10 View FIGURE 11 ). Ovotestis acinose, four acini embedded in last whorls of posterior lobe of digestive gland; hermaphroditic duct long, with a median convoluted seminal vesicle, connecting to base of albumen gland through a small, pouch-like fertilization chamber; spermoviduct morphologically divided into three portions: (1) a proximal whitish, globular, glandular portion into which the fertilization chamber opens distally, (2) a narrow, weakly convoluted channel, and (3) a wide, strongly convoluted and internally folded portion to which the elongate prostate gland adheres; after the separation of the prostate duct into a vas deferens, a free oviduct funnels into the confluence with the bursa duct, both ducts opening into a wider vagina, the extremities of the three ducts covered with a spongy perivaginal gland; vagina adhering to neck wall, the proximal half muscular, smooth outside and ridged inside, the distal portion spongy, apparently glandular, opening into a small, internally smooth genital atrium. Penis almost entirely covered with a thick penial sheath, long and very thin, divided by a constriction into two equally long chambers, with a conspicuous penial caecum proximally, and the distal half internally ridged with pilasters; epiphallus at least twice as thick as the penis where it inserts laterally through a narrow duct; the epiphallus is composed of two distinct long swellings, the proximal portion attached by muscular strands to the edge of the penial sheath (primary epiphallic attachment), the distal portion attached to the penial constriction (secondary epiphallic attachment), thus causing the distal swelling to be almost entirely wrapped by the penial sheath; penial retractor muscle long and thin, inserting on back of the mantle cavity, near the heart region. Spermatophore ( Fig. 12 View FIGURE 12 ) moderately long, thin, anterior portion hooked, with four high, longitudinal ridges, remainder of spermatophore laterally flattened.
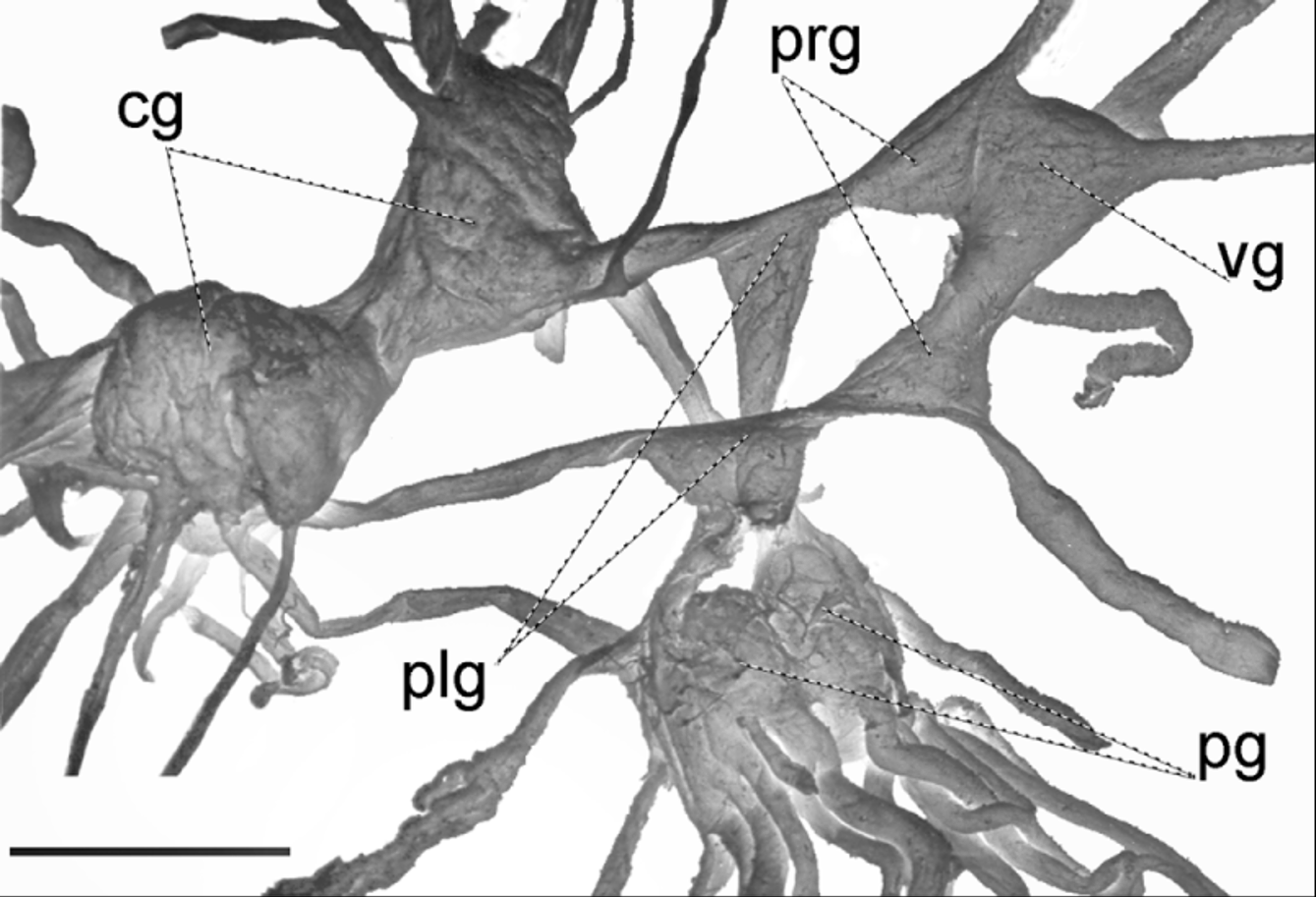
Nervous system ( Fig. 13 View FIGURE 13 ) of the zonitoid type (Bargmann 1930), the cerebral ganglia large, pleural ganglia triangular, left parietal ganglion conspicuous, distinct from visceral ganglion which is fused to right parietal ganglion; right cerebropleural connective very short causing the visceral nerve ring to be lopsided to the right; pedal ganglia nearly fused, slightly smaller than the cerebral ganglia. Right tentacular nerve crossing between male and female organs; penial nerve branching off the right internal lip nerve.
Habitat. Like most consubgenerics, Oxychilus (Drouetia) viridescens lives in shady, moist environments of primary forests, and of secondary forests of Pittosporum , Acacia or Cryptomeria with undergrowth of Tradescantia , preferentially gathering under piles of rocks, fallen logs and dead leaves of Hedychium .
Distribution. The new species is distributed throughout the mountainous region of the island of Santa Maria. Although not common, it was most abundantly collected at the type locality, near Santa Bárbara, and also on the northern and western slopes of Pico Alto.
Remarks. In their first publication, Morelet & Drouët (1857) described most of the new records from their expedition to the Azores without precise locality data. Subsequently, Morelet (1860) provided these data while Drouët (1861) further completed them with his own notes. Morelet (1860) vaguely stated that Helix atlantica lived in most Azorean islands but, apparently, the species description was based on material from São Miguel, for Morelet (1860: 168–169) remarked that it was abundant mainly in this island, while referring to peculiar varieties in Santa Maria and Faial. However, no type locality was assigned to the species. Morelet's (1860) variety “γ var. spectabilis ” from Santa Maria was elevated to specific rank by Milne-Edwards (1885) ( Fig. 14 View FIGURE 14 ), and Riedel (1964) awarded subspecific rank to Morelet's (1860) “β minor ” from Faial and described Oxychilus atlanticus brincki from material collected in 1957 by the Lund expedition from which he selected a holotype ( Fig. 15 View FIGURE 15 A). Riedel (1964) also restricted the type locality of Helix atlantica to the island of São Miguel. Later Riedel (1980) raised the taxa from Santa Maria and Faial to species level. However, three specimens from Santa Maria are marked as syntypes of H. atlantica in the Natural History Museum, London; two of them belong in fact to the new species herein described, the other being Riedel’s (1964) O. (D.) brincki . Hence, due to Riedel’s (1964) taxonomic decisions, the type material for the name Helix atlantica is from a locality where this species does not occur. Therefore, a petition was submitted to the ICZN (Case 3553; Martins et al. 2011) to render those specimens unavailable for nomenclatural purposes. However, the recent discovery of Henri Drouët’s collection and syntypes at the Muséum Jardin des Sciences de Dijon (MJSD) (Cédric Audibert, in litt.), has allowed us to select a lectotype of H. atlantica from São Miguel island, thereby rectifying the anomalous situation created by Riedel (1964).
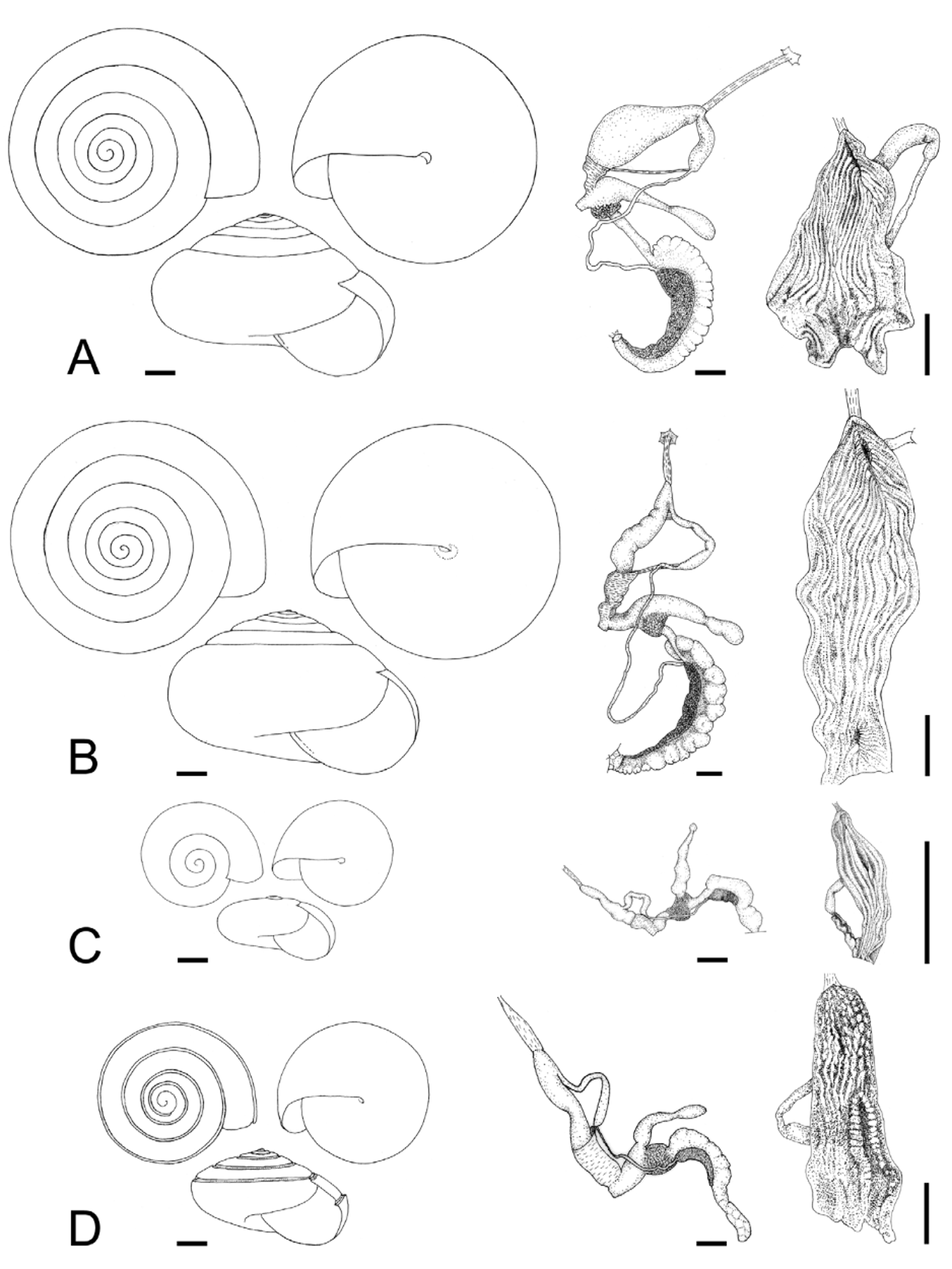
The imperforate Oxychilus are represented in the Açores by two endemic subgenera: Drouetia , present throughout the archipelago, and the monotypic Atlantoxychilus Riedel, 1964 , only recorded from Santa Maria [O. (A.) spectabilis (Milne-Edwards, 1885)]. Three species of Drouetia live in Santa Maria: Oxychilus (D.) brincki , O. (D.) agostinhoi Martins, 1981 and O. (D.) viridescens n.sp ( Figs. 15 View FIGURE 15 , 16 View FIGURE 16 ). Oxychilus (D.) viridescens and O. (D.) brincki are conchologically similar but can be readily distinguished from Oxychilus (D.) agostinhoi , which has a very small, paucispiral, flat shell and from O. (A.) spectabilis which bears a distinct furrow along the suture; the microsculpture of the first whorls is more marked in the new species and in O. (D.) brincki , somewhat fainter in O. (D.) agostinhoi and absent in O. (A.) spectabilis . A more detailed comparison is shown in Table 3.
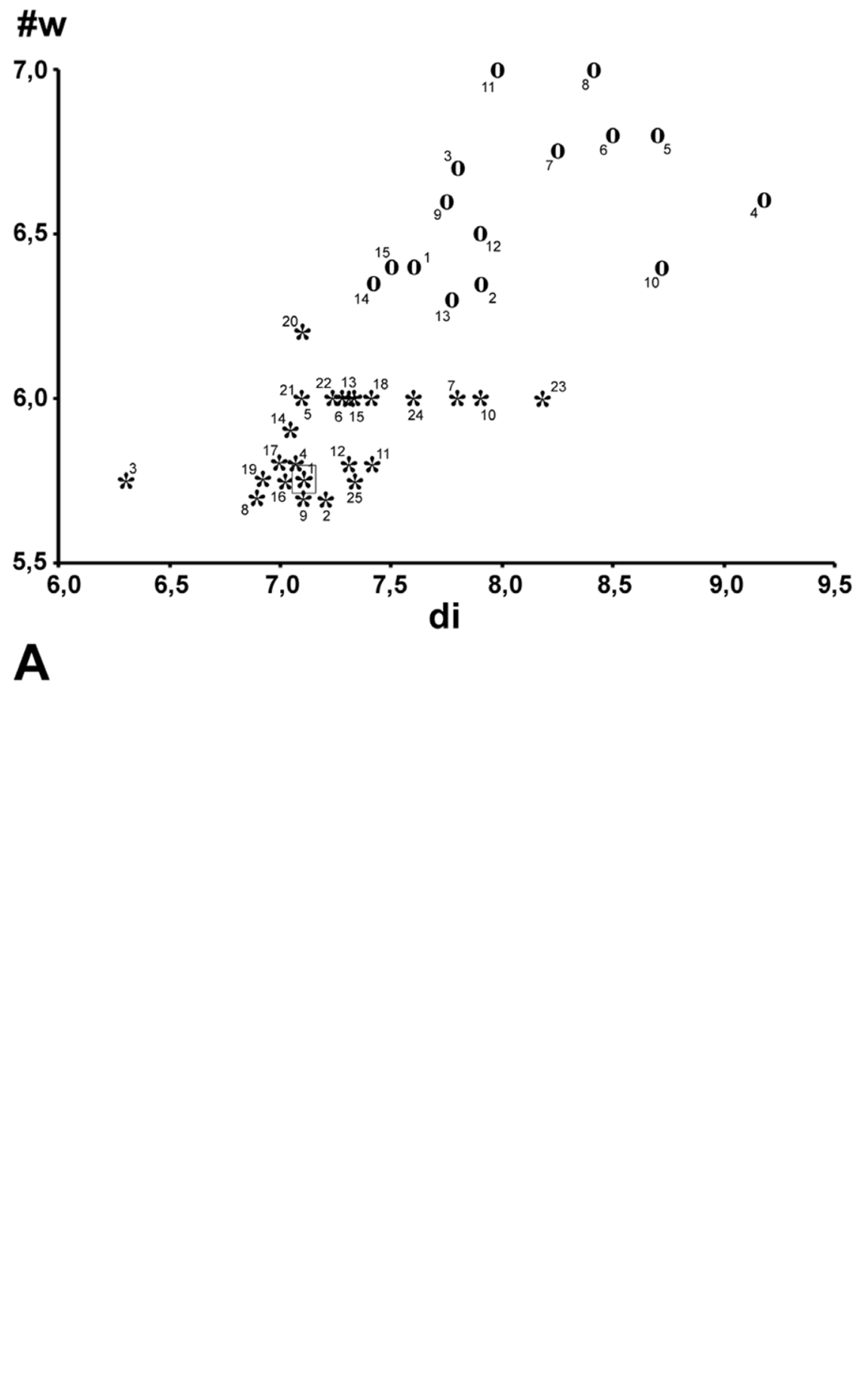
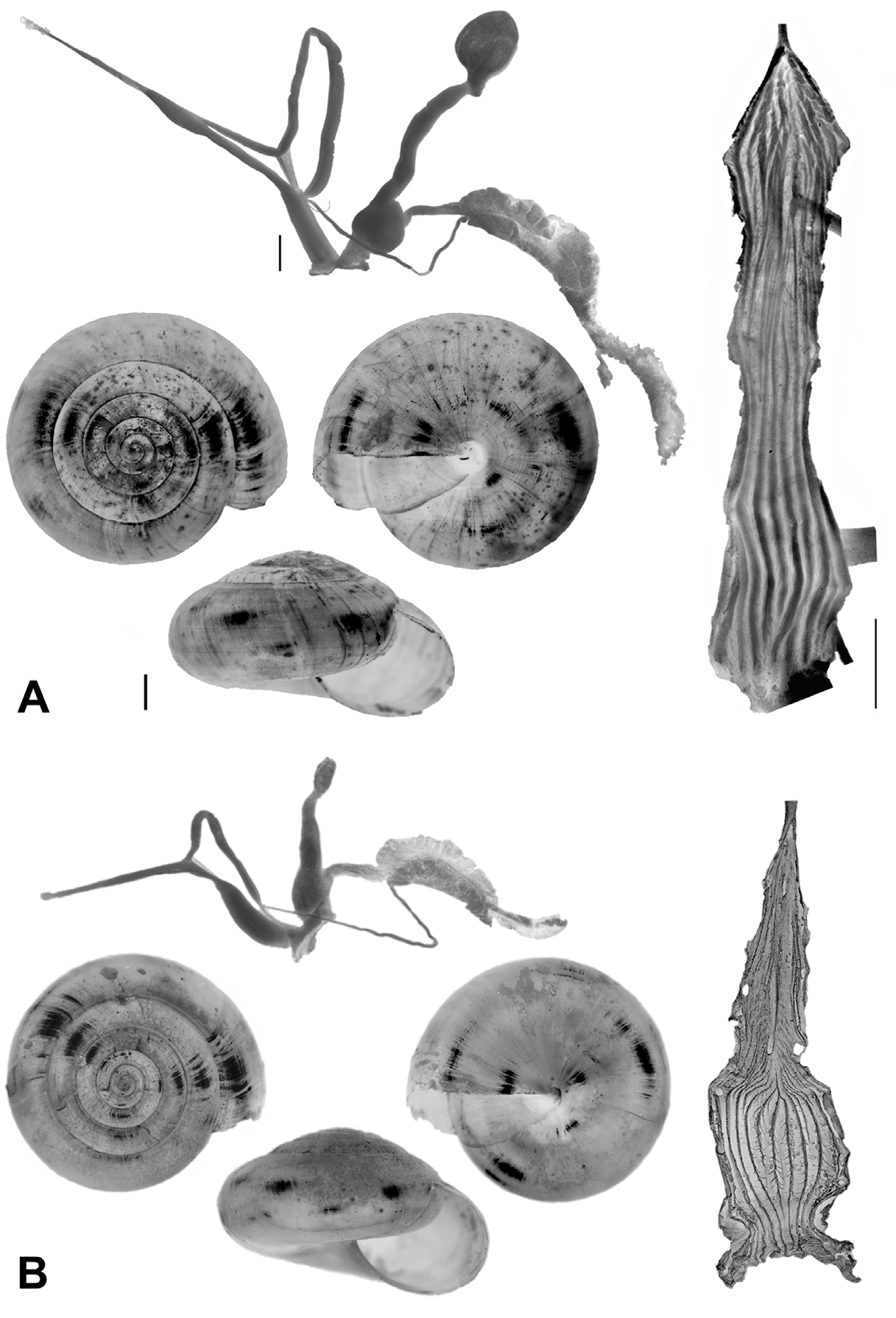
At first sight Oxychilus (Drouetia) viridescens n.sp. could be confused with the sympatric O. (D.) brincki because both species have similar shells. However, a brief survey of simple morphometric characters ( Fig. 2 View FIGURE 2 ; Table 2 View TABLE 2 ) showed that, although with some overlap, the new species has about half a whorl less and is more depressed than O. (D.) brincki ( Fig. 17 View FIGURE 17 ). Because the palatal lip and the columellar lip of the shell run almost at the same plane, the aperture, seen from the bottom, is very narrow in the new species whereas it is extended in O. (D.) brinki. Moreover, fresh shells of the new species exhibit a conspicuous greenish colour whereas the shell of O. (D.) brincki has a more golden-brown pigmentation; the coloration around the umbilical area is grayish in the new species, but it is pinkish in O. (D.) brincki ( Figs. 4 View FIGURE 4 C, D). The colour pattern of the animal in both species shows also some differentiation, with greenish tones and smaller, sparser and whitish blotches on the mantle, as well as a whitish foot predominating in the new species ( Figs. 4 View FIGURE 4 A; 18A), whereas yellowish blotches predominate in the mantle of O. (D.) brincki , and its foot is typically yellowish to orange ( Fig. 4 View FIGURE 4 B). The mantle of O. (D.) agostinhoi may sometimes exhibit a greenish colour, but it is usually redish-brown with some small, whitish spots around the spire and large, black spots restricted to the apertural area, a feature common in the genus; the border of the mantle is black, the neck dark-blue with lighter-blue transverse stripes anteriorly, changing posteriorly to a lighter-blue background strewn with darker spots; foot light-blue, bordered by a dark-blue rim ( Fig. 18 View FIGURE 18 B).
The reproductive system remains the most important source of characters by which the new species can be differentiated; however, care must be exercised when comparing structures, for the degree of relaxation at the time of death may affect the shape and the proportions of the various organs or parts thereof (Martins 1991). Also the degree of development may render some structures more evident at certain times of the year (Rodrigues et al. 1998; Cunha et al. 2001). In this work, only organs that do not depend entirely on the degree of maturation were used (e.g. penis vs. albumen gland) or structures that, although variable in other taxa, were observed to maintain a relatively constant pattern across different preserving situations.
The reproductive system, and in particular the morphology of the penial complex, is very diverse in the four species from Santa Maria. The distal glandular vagina, the epiphallus with double swellings, and the very thin penis of O. (D.) viridescens n.sp. ( Figs. 10 View FIGURE 10 , 11 View FIGURE 11 ) are unique features in the Azorean oxychilids; the muscle strands attaching the distal portion of the epiphallus to the penial constriction are also seen in O. (D.) atlanticus ( Fig. 19 View FIGURE 19 A) and O. (D.) furtadoi Martins, 1989 ( Fig. 20 View FIGURE 20 B), although not as developed as in the new species.
Some variability was found in the morphology of the male organ of O. (D.) brincki . The typical form, from Pico Alto, shows a stout, bulging penial complex and a short, thick epiphallus connected to the penial sheath through a very long, thin muscle, whereas the specimens from Santa Bárbara have a much thinner penis and epiphallus and a shorter muscular attachment of the latter structure. The internal morphology of the penis, however, with many long, homogeneous furrows running from the epiphallic pore, is a reliable indicator of the conspecificity of both populations; this morphology is also unique among Drouetia ( Fig. 17 View FIGURE 17 A, B).
The penis of O. (D.) viridescens n.sp. and that of O. (D.) agostinhoi may exhibit a mid-length constriction (see Martins 1981), whose presence may depend on the degree of relaxation at the time of death. This constriction was never observed in the two other species. Oxychilus (D.) viridescens n.sp. and O. (D.) agostinhoi are also similar in the presence of a few strong pilasters inside the penis ( Figs. 10 View FIGURE 10 ; 11; 16B); this resemblance, however, is probably misleading, for it can be attributed to the narrow penis of the former and to the small size of the latter species.
TABLE 3. Comparison of the morphological and anatomical characters of Oxychilus (Drouetia) viridescens , n.sp. with the related species living in Santa Maria, O. (D.) brincki , O. (D.) agostinhoi and O. Atlantoxychilus ) spectabilis .
Characters O. (D.) viridescens O. (D.) brincki O. (D.) agostinhoi O. (A.) spectabilis
Animalcoloration
Neck color dark blue dark blue deep dark blue, transverse light streaks light pink, darker in front, faint gray
running down toward foot blotches posteriorly
Anterior tentacles faint blue dark blue dark blue, tips lighter light blue
Posterior tentacles dark blue dark blue deep dark blue dark blue
Posterior tentacle retractors entirely blackish entirely blackish entirely blackish dark blue, fading back until half neck
Foot rim dark blue posteriorly only same color as foot sole almost continuously dark blue brownish, interrupted all along foot
Foot sole Whitish green to dark yellow yellowish green to orange whitish pinkish
Collar blackish near pneumostome, fading to blackish near pneumostome, fading to blackish all around pinkish all around pinkish on left side pinkish on left side
Pneumostomal area all blackish pinkish above, dark blue underneath all blackish pink, faint bluish blotch underneath
Mantle color brown pale brown dark yellow to brick red golden yellow
Front blotch black, only on right side black, only on right side black, across front black, only on right side
Mantle blotches light yellow, small, sparse whitish, large, abundant dark yellow, very small, very sparse whitish, large, abundant
Shell
Maximum diameter (mm) 9.0 8.9 5.1 5.5
Number of whorls 6.2 6.8 4.2 5
Spire moderate high flat moderate
Aperture, basal view narrow very extended extended moderately extended
General coloration greenish golden brown greenish golden brown
Umbilical region grayish pinkish whitish golden brown
Sculpture finely grooved spirally finely grooved spirally very finely grooved spirally smooth, w/ furrow
marginal teeth/half row 21 19 13 19
cusps of central tooth central cusp much longer central cusp much longer cusps small, equal central cusp much longer
Mandible slender and curved slender and less curved slender and less curved very slender and straight
Mantlecollar
Right lateral lobe elongate elongate truncate elongate
Posterior canal round, rings tightly compressed round, rings tightly compressed sharply acute, rings convoluted moderately acute, rings tightly
compressed
HermaphroditicReproductiveOrgans
Prostate long long short short
Free oviduct somewhat shorter than vagina much longer than vagina about as long as vagina much shorter than vagina
Base of bursa duct as wide as free oviduct much wide r than free oviduct about as wide as free oviduct about as wide as free oviduct
Relative width of bursa much wider than duct about as wide as duct much narrower than duct about as wide as duct
Perivaginal gland covering bursa duct and oviduct covering oviduct covering bursa duct and oviduct covering oviduct
Distal vagina glandular (yes/no) yes no no no
Penialcomplex
General shape thinner than vagina much thicker than vagina about as thick as vagina about as thick as vagina
Penial sheath very long very short moderately short moderately short
Penial caecum small very small long small
Shape of epiphallus double single single single
Primary epiphallic attachment short long short short
Secondary epiphallic attachment present absent absent absent
Penial constriction present absent present absent
Interior sculpture few furrows many furrows few furrows furrows and papillae Oxychilus (A.) spectabilis has a rather peculiar internal penial morphology: it resembles O. (O.) cellarius (Müller, 1774) by the presence of rows of papillae which coalesce into weak pilasters (Giusti & Manganelli 1997), but it also shows an arch of conspicuous conical papillae ( Figs. 14 View FIGURE 14 ; 16D). In fact, the rows of papillae inside the penis, coalescing into weak pilasters, reminded Riedel (1964) of the situation in Oxychilus s.s. Yet, he dismissed such a relationship because in Atlantoxychilus the papillae are conical, whereas in Oxychilus s.s. they are squamiform. Riedel (1964), however, did not mention the peculiar arch of prominent papillae in Atlantoxychilus . On the other hand, Riedel (1980) did show that the Caucasian O. (Conulopolita) raddei (Boettger, 1879) has a somewhat similar, but more complex structure as that found in O. (A.) spectabilis even though the remaining internal penial surface appeared to be smooth in the Caucasian species. However, the relationships of these two taxa cannot be ascertained on that peculiarity alone.
TABLE 2. Measurements (mm) and counts of various shell characters of specimens of Oxychilus (D.) viridescens (Ov) and Oxychilus (D.) brincki (Ob). di, shell diameter; he, height of the shell; sp, height of the spire; sta / date, station number / date of collection; spc. #, specimen number; # w, number of whorls. For museum abbreviations, see text under “ Types ”.
| spc.# | #w | di | he | sp | sta/date | Observations |
|---|---|---|---|---|---|---|
| Ov1 | 5.75 | 7.12 | 3.83 | 0.65 | 1/(12-12-2008) | holotype (NHMUK 20100658) |
| Ov2 | 5.70 | 7.20 | 3.79 | 0.60 | 1/(22-10-1993) | paratype (NHMUK 20100659a) |
| Ov3 | 5.75 | 6.30 | 3.78 | 0.81 | 1/(22-10-1993) | paratype (NHMUK 20100659b) |
| Ov4 | 5.80 | 7.05 | 3.89 | 0.72 | 1/(22-10-1993) | paratype (MNHN 24265) |
| Ov5 | 6.00 | 7.10 | 3.98 | 0.87 | 1/(22-10-1993) | paratype (USNM 1155712) |
| Ov6 | 6.00 | 7.22 | 4.43 | 0.79 | 1/(22-10-1993) | paratype (MCZ 373862) |
| Ov7 | 6.00 | 7.80 | 4.00 | 0.61 | 1/(22-10-1993) | paratype (IG31765 (MT 2329)) |
| Ov8 | 5.70 | 6.89 | 3.72 | 0.51 | 1/(22-10-1993) | paratype (MP 1011) |
| Ov9 | 5.70 | 7.10 | 3.93 | 0.65 | 1/(22-10-1993) | paratype (DB/UAç-MT 1423) |
| Ov10 | 6.00 | 7.90 | 4.52 | 0.99 | 1/(22-10-1993) | paratype (DB/UAç-MT 1423) |
| Ov11 | 5.80 | 7.41 | 4.07 | 0.70 | 1/(28-03-1996) | paratype (DB/UAç-MT 1424) |
| Ov12 | 5.80 | 7.31 | 4.30 | 0.72 | 1/(28-03-1996) | paratype (DB/UAç-MT 1424) |
| Ov13 | 6.00 | 7.28 | 3.89 | 0.65 | 1/(28-03-1996) | paratype (DB/UAç-MT 1424) |
| Ov14 | 6.00 | 7.05 | 4.25 | 0.97 | 1/(28-03-1996) | paratype (DB/UAç-MT 1424) |
| Ov15 | 6.00 | 7.32 | 4.42 | 0.78 | 6/(02-11-1974) | (DB/UAç-MT 1425) |
| Ov16 | 5.75 | 7.02 | 3.88 | 0.55 | 6/(02-11-1974) | (DB/UAç-MT 1425) |
| Ov17 | 5.80 | 7.00 | 3.76 | 0.62 | 6/(02-11-1974) | (DB/UAç-MT 1425) |
| Ov18 | 6.00 | 7.40 | 4.20 | 0.95 | 6/(02-11-1974) | (DB/UAç-MT 1425) |
| Ov19 | 5.75 | 6.92 | 3.91 | 0.64 | 6/(02-11-1974) | (DB/UAç-MT 1425) |
| Ov20 | 6.20 | 7.10 | 4.28 | 0.72 | 6/(02-11-1974) | (DB/UAç-MT 1425) |
| Ov21 | 6.00 | 7.10 | 4.18 | 0.70 | 6/(21-10-1993) | (DB/UAç-MT 1426) |
| Ov22 | 5.90 | 7.22 | 4.22 | 0.71 | 2/(13-06-1990) | (DB/UAç-MT 1427) |
| Ov23 | 6.00 | 8.18 | 4.86 | 0.74 | 5/(30-03-1996) | (DB/UAç-MT 1428) |
| Ov24 | 6.00 | 7.60 | 3.85 | 0.61 | 1/(22-10-1993) | paratype (DB/UAç-MT 1423) |
| Ov25 | 5.75 | 7.33 | 4.07 | 0.89 | 1/(28-03-1996) | paratype (DB/UAç-MT 1448) |
| Ob1 | 6.40 | 7.60 | 4.65 | 0.95 | 2/(13-06-1990) | (DB/UAç-MT 1442) |
| Ob2 | 6.35 | 7.90 | 4.53 | 0.95 | 2/(13-06-1990) | (DB/UAç-MT 1442) |
| Ob3 | 6.70 | 7.80 | 4.85 | 1.04 | 10/(13-06-1990) | (DB/UAç-MT 1443) |
| Ob4 | 6.60 | 9.18 | 5.50 | 1.11 | 5/(30-03-1996) | (DB/UAç-MT 1444) |
| Ob5 | 6.80 | 8.70 | 5.59 | 1.32 | 5/(13-06-1986) | (DB/UAç-MT 1445) |
| Ob6 | 6.80 | 8.50 | 5.00 | 0.90 | 7/(12-06-1986) | (DB/UAç-MT 1446) |
| Ob7 | 6,75 | 8.25 | 5.19 | 0.95 | 7/(21-06-1994) | (DB/UAç-MT 1447) |
| Ob8 | 7.00 | 8.41 | 5.10 | 1.15 | 1/(28-03-1996) | (DB/UAç-MT 1448) |
| Ob9 | 6.60 | 7.75 | 4.98 | 1.31 | 1/(28-03-1996) | (DB/UAç-MT 1448) |
| Ob10 | 6.40 | 8.72 | 4.76 | 0.98 | 1/(28-03-1996) | (DB/UAç-MT 1448) |
| Ob11 | 7.00 | 7.98 | 5.11 | 1.48 | 1/(28-03-1996) | (DB/UAç-MT 1448) |
| Ob12 | 6.50 | 7.90 | 4.80 | 0.92 | 1/(28-03-1996) | (DB/UAç-MT 1448) |
| Ob13 | 6.30 | 7.77 | 4.57 | 1.03 | 1/(28-03-1996) | (DB/UAç-MT 1448) |
| Ob14 | 6.35 | 7.42 | 4.65 | 0.81 | 1/(28-03-1996) | (DB/UAç-MT 1448) |
| Ob15 | 6.40 | 7.50 | 4.60 | 0.99 | 1/(28-03-1996) | (DB/UAç-MT 1448) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |