Ospreyella mayottensis Simon, Hiller, Logan & Mottequin, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4613.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:A6591BD1-5729-49B2-980B-F1804FE28D3F |
|
persistent identifier |
https://treatment.plazi.org/id/038F87F8-FF81-FFD6-FF23-F2059D58FCBC |
|
treatment provided by |
Plazi |
|
scientific name |
Ospreyella mayottensis Simon, Hiller, Logan & Mottequin |
| status |
sp. nov. |
Ospreyella mayottensis Simon, Hiller, Logan & Mottequin View in CoL , sp. nov.
Table 3; Text-Figs. 2 View TEXT-FIGURE 2 , 6–7; Pl. 5, Figs. 1–2; Pl. 6, Figs. 1–8; Pl. 7, Figs. 1–6
? 1987 Lacazella mauritiana Dall, 1920 View in CoL : Zezina, p. 56, tab. 1.
Holotype. MNHN-IB-2017-179 ( Text-Fig. 2 View TEXT-FIGURE 2 ; Pl. 5, Fig. 1), a fully female adult articulated specimen, which has been opened for the study of the brachidium.
Paratypes. MNHN-IB-2017-181 (Pl. 6, Fig. 1), MNHN-IB-2017-180 (Pl. 5, Fig. 2), MNHN-IB-2017-182 (Pl. 6, Fig. 2), and MNHN-IB-2017-183 (Pl. 6, Fig. 3), articulated specimens. MNHN-IB-2017-184 (Pl. 6, Fig. 4), dorsal valve of opened male specimen. MNHN-IB-2017-191 (pl. 7, fig. 3), MNHN-IB-2017-192 (Pl. 7, Fig. 4), MNHN- IB-2017-193 (Pl. 7, Fig. 5), and MNHN-IB-2017-194 (Pl. 7, Fig. 6), disarticulated or opened specimens used for ontogenetic study. MNHN-IB-2017-185 (pl. 6, Fig. 5), MNHN-IB-2017-187 (Pl. 6, Fig. 7), MNHN-IB-2017-188 (Pl. 6, Fig. 8), MNHN-IB-2017-186 (Pl. 6, Fig. 6), MNHN-IB-2017-190 (Pl. 7, Fig. 2), and MNHN-IB-2017-189 (Pl. 7, Fig. 1), opened specimens used for detailed views of specific parts of the shell. MNHN-IB-2017-196 (Text-Fig. 6.2) and MNHN-IB-2017-195 (Text-Fig. 6.1), specimens used for critical point analyses and study of the lophophore. Morphometric measurements of holotype and paratypes are presented in Table 3.
Etymology. The name of the species is the Latin translation of the Island of Mayotte.
Type locality. Department of Mayotte ( France). Submarine cave at 23 m depth situated at La Passe bateau off the south-west coast of the Island of Mayotte in the second coral reef barrier (Latitude: -12.9776, Longitude +44.9827).
Additional material. Material collected from the dried sieved sediment: 55 articulated specimens, 264 isolated dorsal and 40 isolated ventral valves. Material collected from samples of the walls of the cave and preserved in ethanol for study of the lophophore and future DNA studies: ten specimens.
Diagnosis. Medium-sized thecideide brachiopod (adult shell length around 3–5 mm). Female shells are as large as male shells and with similar complete ontogenetic development (gonochoristic species). Shell whitish, ventribiconvex, rectimarginate anteriorly.
Ventral valve with well-developed, triangular, convex rugideltidium, anterior part with numerous irregular cavities. Faint anterior sulcus present in some specimens, absent in others. Median ridge in the ventral valve present. Ventral valve floor with two types of spines. Hemispondylium formed by two inwardly concave, pointed, lateral prongs and a prominent median myophragm; no supporting structure of the hemispondylium is observed.
Dorsal valve with narrow and straight median ramus with subparallel frilled margins and concave ventral surface; ramuli much wider than median ramus, strongly concave with weakly frilled margins. Anterior median depression clearly developed and moderately wide. Anterior margin and peripheral ridge slightly tuberculated. Minor interbrachial lobes asymmetrical (one lobe always longer than the other one), weakly inwardly concave to straight, never furcated. Major intrabrachial lobes relatively regular, smooth with finely denticulate margins. Lophophore ptycholophous.
Diagnose. Brachiopode thécidéide de taille moyenne (les adultes ont une longueur de 3–5 mm). Les coquilles de spécimens femelles ou mâles ont une taille identique et un développement ontogénétique similaire. La coquille est blanchâtre, ventri-biconvexe avec une commissure rectimarginée.
La valve ventrale a une interarea triangulaire très développée avec un rugideltidium convexe dont la partie antérieure laisse apparaître de nombreuses cavités irrégulières. Un faible sulcus antérieur est parfois présent, parfois non. Le fond de la valve ventrale possède une crête médiane. Deux structures épineuses différentes se rencontrent sur le fond de la valve ventrale. L’hémispondylium est constitué de deux lames, concaves intérieurement dont l’extrémité est pointue et qui sont réunies par un solide myophragme médian; l’hémispondylium est dépourvu de structures de support.
La valve dorsale a un ramus médian étroit, long nettement concave ventralement, avec des marges subparallèles épineuses. Les ramuli sont nettement plus larges que le ramus médian et ont marges faiblement épineuses. La dépression antérieure est présente et assez large. La bordure antérieure de la crête périphérique est légèrement tuberculée. Les lobes interbrachiaux mineurs sont asymétriques (un lobe toujours plus long que l’autre), plutôt tout droit ou très faiblement concave intérieurement et ne sont jamais divisés. Les lobes intrabrachiaux majeurs sont très réguliers, subcirculaires, lisses avec des marges très finement denticulées. Le lophophore est ptycholophe.
Description. External characters. The shell is relatively large for the genus ( Table 3), reaching a width of about 5 mm ( Table 3) and with a variable outline depending on the substrate to which the shell is fixed. It can have a drop-like aspect (Pl. 5, Fig. 2a; Pl. 6, Fig. 1a) but often it has an apple-shaped outline (Pl. 5, Fig. 1a; Pl. 6, Fig. 2). The maximum width (W) is at the mid-dorsal valve. The value of the length to width (L/W) ratio is constant and the shell keeps its outline unchanged during growth (Text-Fig. 7). The whitish shell is slightly longer than wide and is mainly cemented to the substrate by the posterior part of the ventral valve. Due to this type of attachment, the ventral part of the shell is often distorted (Pl. 5, Fig. 1d; Pl. 6, Fig. 1b, 1d). Rarely, the fixation of the shell occurs on its lateral part (Pl. 6, Fig. 2), a situation illustrating the strong influence of the substrate on the external shape of the shell.
The shell is strongly ventribiconvex (Pl. 5, Fig. 1b; Pl. 6, Fig. 1b), the dorsal valve being relatively flat, lid-like, except for its median part, which is slightly convex. During growth, the thickness of the shell increases relatively to its width, but the value of the thickness to width ratio (T/W) is not significantly modified (Text-Fig. 7). In dorsal view, the shell is sometimes cordiform as its anterior commissure is emarginate. In this case, a shallow median sulcus can develop at the anterior margin of the ventral valve (Pl. 5, Fig. 1a, 1c; Pl. 6, Fig. 1a, 1c) but this character is frequently absent. In lateral view, the entire shell is lifted from the substrate anteriorly (Pl. 5, Fig. 1b; Pl. 6, Fig. 1b), its anterior part tending to be in a more elevated position than the posterior one. Such a geniculation of the ventral valve is common in thecideide brachiopods as illustrated by Pajaud (1970, p. 219, fig. 130A). The anterior commissure is rectimarginate in anterior view (Pl. 5, Fig. 1c; Pl. 6, Fig. 1c). The lateral commissure is variable but generally concave dorsally.
TEXT-FIGURE 6. Illustration of the lophophore in juvenile and adult specimens of Ospreyella mayottensis sp. nov. from “La Passe bateau” off the south-west coast of Mayotte Island. (1a–d) MNHN-IB-2017-195 (paratype): a juvenile dorsal valve interior in plan and oblique lateral views (schizolophous lophophore including between 45–48 tentacles), close-up of the posterior part of the valve with the muscles, and detailed view of the right side of the lophophore. (2a–e) MNHN-IB-2017-196 (paratype): an adult disarticulated specimen. a–d. Dorsal valve interior showing the ptycholophous lophophore (around 140 tentacles) in plan, oblique anterior and oblique anterolateral views. e. Ventral valve interior in oblique anterior view (critical point dried) showing the two pads covering the gonads (on the right side the pad is still placed correctly (pad) whereas on the left side, the pad has moved laterally).
The ventral valve has a relatively long and strongly produced beak with a flat triangular interarea ornamented with numerous fine regular growth lines, parallel to the hinge line. A raised triangular, transversely convex rugideltidium is developed (Pl. 5, Figs. 1a, 1d, 2a–e; Pl. 6, Figs. 1a, 1d, 2, 5a, 7). The rugideltidium is quite long and can represent up to 30 % of total shell length. The dorsal surface of the rugideltidium is flatly-convex, irregular and growth lines are not very distinct (Pl. 6, Fig. 1a). The interarea and the convex rugideltidium are usually straight but often they may be strongly curved to the right or to the left. The anterior part of the rugideltidium is normally smooth but, if this surface is eroded, the internal structure of the rugideltidium becomes visible and is made of numerous irregular cavities that are not endopunctae (Pl. 5, Fig. 2b; Pl. 6, Fig. 5c).
Subparallel undulating growth lines are visible on the external ventral shell surface and their thickness is variable (Pl. 6, Fig. 1c).
The lid-like dorsal valve is always wider than long, oval in shape and often with an emarginate anterior commissure (Pl. 5, Fig. 1a; Pl. 6, Figs. 1a, 2). On the dorsal valve surface, the growth lines are indistinct and irregular. The protegulum is prominent and has a granular surface (Pl. 5, Fig. 2b; Pl. 6, Fig. 8).
The hinge line is straight and always narrower than the maximum width of the valve ( Table 3). The length of the hinge line increases during growth as indicated by the values of the width of the hinge to the width of the shell (WH/W) ratio ( Table 3, Text-Fig. 7).
Most of the morphological characters show a linear variation when compared to value of the width of the shell (Text-Fig. 7). However, the best fitting relation between the length of the interarea and the width of the shell is an exponential variation (Text-Fig. 7). This explains the big difference between the beak length in juveniles and in adult specimens ( Table 3).
Internal shell characters. The ventral valve has a drop-like, slightly bilobed and cordiform outline due to its emarginate anterior commissure (Pl. 5, Figs. 1l, 2c; Pl. 6, Fig. 5a). All around the commissure is a smooth peripheral flange, which seals the shell when the valves are closed. The commissure is limited internally by a peripheral rim with a smooth external side and a row of more or less regular, strong tubercles on the internal side (Pl. 5, Fig. 2c, 2e; Pl. 6, Figs. 5a).
A wide median ridge is visible in the middle of the ventral valve floor. This median ridge separates the two deep gonad pits placed in the posterior part of the valve (Pl. 5, Fig. 2c, 2e; Pl. 6, Fig. 5a). The valve floor shows endopunctae and is roughly granulated. At high magnification the surface appears to be ornamented with small spinous asperities (Pl. 7, Fig. 1a). At higher magnification, two kinds of spines are discovered: there are small narrow cylindrical spines (less numerous) and there are wider conical structures that are more numerous (Pl. 7, Fig. 1a–b). The role of such very peculiar ornamentation remains unknown.
The strong cyrtomatodont teeth are short, relatively thick, and reinforced with secondary shell material (Pl. 6, Fig. 5b). The hemispondylium consists of two pointed lateral prongs and a prominent median lamellar myophragm (Pl. 5, Figs. 1l, 2e: Pl. 6, Fig. 5b). A septum supporting the hemispondylium is not observed in adult or young specimens.
In specimens collected alive and treated using the critical point method, two calcitic oval pads covering the gonads are visible in the posterior part of the ventral valve floor (Text-Fig. 6.2e). In this illustration, the right plate is still in its correct position whereas the left one has moved laterally. The external edge of this calcitic plate is denticulate. In female specimens a median brood pouch containing the larvae is situated between the gonad pads.
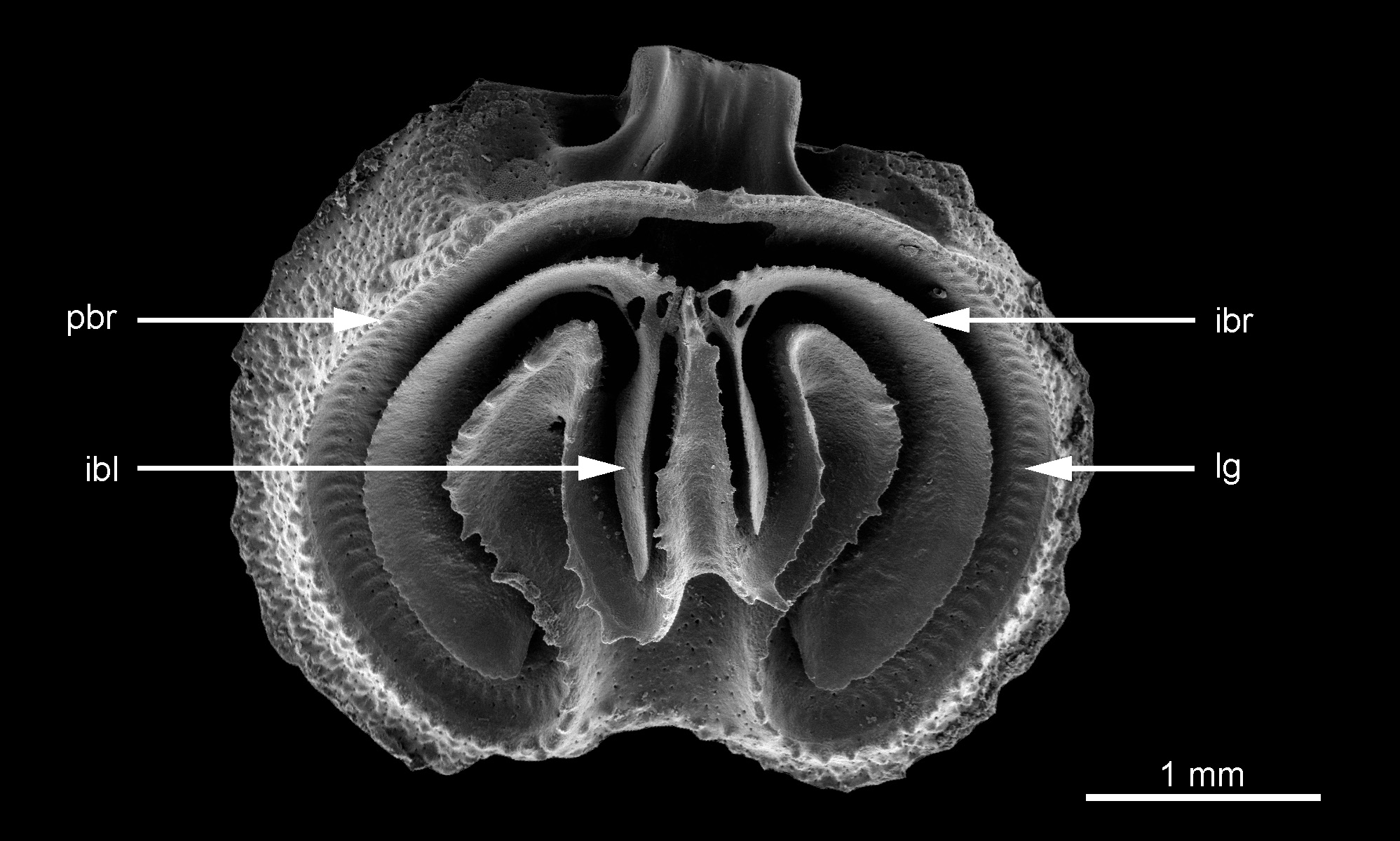
The dorsal valve in ventral view is widely oval, usually with an emarginate anterior commissure (Pl. 5, Fig. 1e) but sometimes the valve is not emarginate (Pl. 6, Fig. 4a). In completely preserved specimens a narrow smooth, flat flange is present along the commissure (Pl. 6, Fig. 4a–d). The peribrachial ridge is covered with numerous tubercles that can have a highly variable outline. They can be subspherical with a moderate size and a cup-like aspect (Pl. 5, Fig. 1i) or strong irregular tubercles (Pl. 6, Fig. 4b–c) and sometimes showing a mixed ornamentation (Text-Fig. 6.2a–b).
The dental sockets are strong, formed by curved inner socket ridges and totally flat, depressed, outer socket ridges (Pl. 5, Fig. 1e, j–k; Pl. 6, Fig. 4d). The trilobed cardinal process (Pl. 5, Fig. 1k; Pl. 6, Fig. 4d) has a wide smooth ventral surface and is curved dorsally. Secondary shell material thickens the lateral parts (Pl. 5, Fig. 1k; Pl. 6, Fig. 4d). The development of the median lobe is most prominent in its posterior part.
Wide drop-like lateral adductor muscle scars are clearly developed on either side of the cardinal process (Pl. 5, Fig. 1k; Pl. 6, Fig. 4d). Between the cardinal process and the brachial bridge, a heart-shaped visceral foramen (Pl. 5, Fig. 1k; Pl. 6, Fig. 4d), free of a calcitic pole, is apparent. The diductor muscle scars are sometimes visible on the posterior margin of the cardinal process (Pl. 6, Fig. 4d) but they are weakly defined.
The shell structures developed in the dorsal valve are slightly raised towards the posterior part of the valve (Pl. 7, Fig. 4c). A thin and relatively narrow brachial bridge (Pl. 5, Fig. 1k) is built by the fusion of the posterior parts of the peribrachial ridge margin (similar to a fusion of crural processes as proposed by Logan (2008, p. 411)). This brachial bridge is thin and remains complete in male specimens (Pl. 6, Fig. 4a, 4d) but is interrupted by a marsupial notch in female specimens (Pl. 5, Fig. 1e, 1g, 1i-k). The marsupial notch is a small subcircular hole open on the ventral side of the brachial bridge. The posterior face of the marsupial notch exhibits a small platform with a small convex median ridge (Pl. 5, Fig. 1g; Pl. 7, Fig. 2a–b) and a short posterior point. The inner surface of the marsupial notch exhibits the muscle scars of the pair of specialized tentacles (Pl. 5, Fig. 1g) that are related to the development of the embryos in the brood pouch (Text-Fig. 6.2d).
The lophophore is attached to the lophophore groove that follows the internal side of the peribrachial ridge, the external sides of the ramuli and the lateral sides of the median ramus (Pl. 5, Fig. 1e; Pl. 6, Fig. 4a) and the lophophore muscle scars are clearly defined by numerous parallel imprints.
The margins of the intrabrachial ridges exhibit short spines (Pl. 5, Fig. 1e–f) that can be very weakly developed in some specimens (Pl. 6, Fig. 4a–b).
In their posterior part, the margins of the major intrabrachial lobes are connected by means of the jugum (Pl. 5, Fig. 1f; Pl. 6, Fig. 4a). The major intrabrachial lobes are widely developed in this species and have a very regular subcircular outline. Their ventral surfaces are smooth but their external margins are finely and regularly denticulate.
Two subparallel minor interbrachial lobes extend anteriorly from either side of the jugum. These are relatively long and thick with rather sharp edges; their ventral surfaces are granular (Pl. 5, Fig. 1f). They are never observed to be divided or furcated in any of our specimens. The development of the minor interbrachial lobes is asymmetrical (Pl. 5, Fig. 1f; Pl. 6, Fig. 4a), with the longer lobe indifferently the right or the left one.
The pointed median ramus has a strongly concave upper surface, a relatively long and narrow triangular outline with frilled lateral margins and is connected posteriorly by the small jugum (Pl. 5, Fig. 1f) to the intrabrachial ridge. The median ramus remains strongly concave throughout ontogeny and it is never filled by secondary shell material. The length of the median ramus is variable; it originates from the middle or sometimes a little more anterior part of the valve. The median ramus is attached to the valve floor by a triangular base developed in the middle of the anterior part of the valve (Pl. 6, Fig. 4b).
Two strong lateral ramuli are well-developed. The ramuli are wider than the median ramus and their upper surfaces are deeply concave, a morphology maintained throughout ontogeny. They are never thickened by deposition of secondary shell material. The lateral margins of the ramuli are ornamented with several irregular spines on both sides.
The median anterior depression is relatively narrow, its anterior margin being limited by the tuberculated peripheral rim. The ascending apparatus and the descending apparatus are developed symmetrically (Pl. 5, Fig. 1e; Pl. 6, Fig. 4a).
The adult lophophore is ptycholophous with approximately 160 tentacles observed in an adult female specimen (Text-Fig. 6.2b). At the trocholophous stage, the lophophore possesses 27 tentacles ( Simon & Hoffmann 2013, pl. 4, fig. 1) but this number increases rapidly and 46–50 tentacles are observed in a young schizolophous developmental stage (Text-Fig. 6.1a). In male specimens, the lophophore is developed regularly whereas in females, the lophophore is interrupted posteriorly, allowing the development of a pair of specialized median tentacles (Text-Fig. 6.2a, c). A complete ontogeny of the lophophore is not available as trocholophe and other intermediate stages of growth were not found in our limited material preserved in ethanol.
Comparison with other species of Ospreyella . Ospreyella mayottensis sp. nov. is directly distinguished from O. depressa Lüter (in Lüter et al. 2003) and from O. maldiviana Logan, 2005 by its minor interbrachial lobes which are never furcated as they are in the two other species.
The external aspect of O. palauensis Logan, 2008 is more subcircular. O. palauensis is distinct from O. mayottensis sp. nov. by its minor interbrachial lobes which are stronger and straighter in the latter. In O. palauensis they have also a tendency to furcation. The median ramus in O. mayottensis sp. nov. is straighter, regularly triangular and longer than in O. palauensis where it is shorter and with a much more variable outline. Moreover, the median ramus can be divided in O. palauensis when furcation of the minor interbrachial lobes occurs.
The Australian Ospreyella sp. from Lizard Island is known from very few specimens (Hoffmann et al., 2009). At first glance the major intrabrachial lobes in Ospreyella sp. have more strongly spinous external margins and the median ramus is rather shorter than in O. mayottensis sp. nov. The ramuli of Ospreyella sp. are poorly developed but the specimens illustrated by Hoffmann et al. (2009) are maybe still at a juvenile stage of growth and do not allow for an accurate comparison.
O. mutiara Simon & Hoffmann, 2013 View in CoL has a more subrectangular outline for its dorsal valve. The major intrabrachial lobes are relatively narrower. The median ramus and the ramuli in O. mutiara View in CoL are thickened by accretion of secondary shell material through ontogeny. The median ramus and the ramuli have a similar width and the margins of the ramuli are strongly and regularly spinous ( Simon & Hoffmann 2013, pl. 5). As O. mutiara View in CoL is hermaphroditic with a protandry, males are much smaller than females. In O. mayottensis View in CoL sp. nov. the dorsal valve is more subcircular. The major intrabrachial lobes are relatively larger and wider. The median ramus and the ramuli are very different, the median ramus being narrow, whereas the ramuli are very wide. Both are markedly concave and not thickened through ontogeny. O. mayottensis View in CoL sp. nov. is gonochoristic and both shells of male and female are of the same size.
Ospreyella View in CoL sp. collected from Europa Island ( Mozambique Channel, off south-west Madagascar) displays some similarities with O. mayottensis View in CoL sp. nov. ( Simon & Hoffmann 2013, pl. 8) but the external aspect of O. sp. is more widely oval with a much shorter interarea ( Simon & Hoffmann 2013, pl. 8, figs. 1a–b, 2). The median ramus is much more variable in outline in Ospreyella View in CoL sp. with more asymmetrical structures of the apparatus descendens and ascendens. A tendency to furcation of the minor interbrachial lobes is visible in Ospreyella View in CoL sp. from Europa Island (see Simon & Hoffmann, 2013: plate 8, fig. 5), a character that is never observed in O. mayottensis View in CoL sp. nov. The latter is also the simplest and the most subsymmetrical species with its regular subcircular major intrabrachial lobe.
The lacazelline material from Secteur de Poindimié (stn. 830) in New Caledonia, collected at 105–110 m depth has been wrongly assigned by Bitner (2010, p. 654, fig. 6) to the genus Lacazella Munier-Chalmas, 1880 View in CoL . This genus has a Mediterranean–Caribbean–Atlantic distribution and it is not present in the Pacific ( Simon & Hoffmann 2013, p. 430). This has been shown by both morphological and molecular approaches. This material should be attributed to an unidentified Ospreyella View in CoL species. The wide anterior depression and the median ramus emerging directly from the middle of the valve floor and not supported by a septum in these specimens confirm this proposition. It is difficult to compare accurately O. mayottensis View in CoL sp. nov. with this New Caledonian material as its preservation is not excellent. If a sexual dimorphism exists in this species, it cannot be pointed out, as all the brachial bridges are broken. However, the median ramus seems to be sometimes as wide as the ramuli ( Bitner 2010, fig. 6D–E) or sometimes much more reduced ( Bitner 2010, fig. 6H). Such a wide variation is not visible in O. mayottensis View in CoL sp. nov. The minor interbrachial lobes are also shorter in the New Caledonian material.
Shell ontogeny. The shell ontogeny of O. mayottensis sp. nov. follows a lacazelline model. It is very similar to the ontogeny already studied for other species, such as O. palauensis Logan, 2008 and O. mutiara Simon & Hoffmann, 2013 . Water movements in the submarine cave weaken the juvenile shells and it has been difficult to find perfectly intact juveniles. However, the main steps of the ontogeny are illustrated in Pl. 7, Figs. 3-6.
A V-shaped structure consisting of two divergent spikes appears in the middle of the valve floor. The two spikes are separated from each other at their bases. The lateral parts of the peribrachial ridge start to develop as small tubercles (Pl. 7, Fig. 3). The brachial bridge is not completely built.
The median ramus emerges in front of the divergent spikes and it develops anteriorly. The two spikes fuse together at their base. The two divergent processes form the beginning of the major intrabrachial lobes. Initially, these grow posteriorly, then bend laterally and turn anteriorly forming the first stages of the apparatus descendens ( Backhaus 1959). These early major brachial lobes appear as two ears with a quite irregular outline (Pl. 7, Fig. 4a–c). Later, the major intrabrachial lobes present an “M”-shaped structure with its extremities slightly widened, giving them a spoon-like aspect.
The peribrachial ridge becomes better developed with more numerous coalescent tubercles and a peripheral flange is clearly visible (Pl. 7, Fig. 4c). Normally, the brachial bridge should be fully developed but a well-preserved specimen has not been found.
The median ramus remains quite small and a submedian elongated shell structure, consisting of two faint ridges, emerges at the anterior base of the median ramus and reaches the anterior part of the valve (Pl. 7, Fig. 5b). The ridges limit the future development of the median depression. The median ramus, which appears as a pointed knob placed at the posterior part of the submedian ridges (Pl. 7, Fig. 5b–c), is related with the jugum, which is connected with the growing major intrabrachial lobes. These new structures are raised from the dorsal valve floor.
TEXT-FIGURE 7. Scatterplots of morphometric measurements of Ospreyella mayottensis sp. nov. Abbreviations: L, length; W, width; LDV, length of dorsal valve; T, thickness; Lint, length of the interarea, Wint: width of hinge line or interarea. Relationships between ratios L/W and width, LDV/W and width, T/W and width, Lint/W and width and Wint/W and width. Linear regression and regression coefficient (R²) indicated except for the graph between Lint and width for which an exponential regression is fitting better. The regression coefficient (R²) is indicated. N is the number of specimens measured.
The ramuli also develop at this stage of growth and they are already wider than the precursor of the median ramus (Pl. 7, Fig. 5b). The brachial bridge is clearly visible and no calcitic pole is produced as is the case in lacazelline thecideides. The tubercles defining the peribrachial ridge are more or less completely fused (Pl. 7, Fig. 5b–c).
The ventral valve shows all the adult characters as the hemispondylium and the rugideltidium are completely formed (Pl. 7, Fig. 5a).
Later, the major intrabrachial ridges reach nearly their adult subcircular outline with a smooth ventral concave surface and finely denticulate margins. The minor interbrachial lobes grow progressively (Pl. 7, Fig. 6a: the left minor interbrachial lobe has been broken). The anterior depression is achieved. The wide ramuli extend and they have frilled margins. The median ramus, remains narrow but it grows in length (Pl. 7, Fig. 6a–c).
The ventral valve floor becomes more spinous with the two types of spines: several narrow cylindrical spines and more numerous wide conical spines (Pl. 6, Fig. 6d).
Structure of the lophophore. After the earliest stage, which is a trocholophe type ( Simon & Hoffmann 2013, pl. 4, fig. 1), the lophophore becomes schizolophous (Text-Fig. 6.1a–b, d). The number of tentacles increases rapidly as it reaches around 45–48 tentacles at this stage of growth.
At the adult growth stage, the lophophore becomes ptycholophous (Text-Fig. 6.2a–d). The number of tentacles is around 140. The filaments are not furcated and seem to have a smooth surface. The lophophore at this stage of growth is attached along the internal side of the peribrachial ridge and along the external sides of the median ramus. The internal parts of the median ramus and of the ramuli are free from the lophophore and are used to facilitate water circulation.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Thecideoidea |
|
Family |
|
|
SubFamily |
Lacazellinae |
|
Genus |
Ospreyella mayottensis Simon, Hiller, Logan & Mottequin
| Simon, Eric, Hiller, Norton, Logan, Alan, Theuerkauff, Dimitri & Mottequin, Bernard 2019 |
O. mayottensis
| Simon & Hiller & Logan & Theuerkauff & Mottequin 2019 |
O. mayottensis
| Simon & Hiller & Logan & Theuerkauff & Mottequin 2019 |
O. mayottensis
| Simon & Hiller & Logan & Theuerkauff & Mottequin 2019 |
O. mayottensis
| Simon & Hiller & Logan & Theuerkauff & Mottequin 2019 |
O. mayottensis
| Simon & Hiller & Logan & Theuerkauff & Mottequin 2019 |
O. mayottensis
| Simon & Hiller & Logan & Theuerkauff & Mottequin 2019 |
O. mutiara
| Simon & Hoffmann 2013 |
O. mutiara
| Simon & Hoffmann 2013 |
O. mutiara
| Simon & Hoffmann 2013 |
Ospreyella
| Luter & Worheide 2003 |
Ospreyella
| Luter & Worheide 2003 |
Ospreyella
| Luter & Worheide 2003 |
Ospreyella
| Luter & Worheide 2003 |
Lacazella mauritiana
| Dall 1920 |
Lacazella
| Munier-Chalmas 1880 |