Oliera argentinana Brèthes, 1916
|
publication ID |
https://doi.org/ 10.5281/zenodo.282899 |
|
DOI |
https://doi.org/10.5281/zenodo.5696597 |
|
persistent identifier |
https://treatment.plazi.org/id/03CB2C50-F903-A41F-FF05-FD15AD9EFAEF |
|
treatment provided by |
Plazi |
|
scientific name |
Oliera argentinana Brèthes, 1916 |
| status |
|
Oliera argentinana Brèthes, 1916
Figs. (1–10)
Material examined. Data are listed as they are found in the labels. Lectotype: 3, B. Aires, Argentina, 25 XI. 915, J. B., pinned, genitalia on slide 4477, D. R. Davis ( MACN) [present designation]. Guaritas, Caçapava do Sul, Rio Grande do Sul (RS), Brazil, 30°50’15”S, 53°30’04”W, November 2007, G.R.P.Moreira & G.L. Gonçalves colls., 7ƤƤ, 83, pinned ( LMCI 11-3-24 to 38), reared from galls collected on Schinus polygamus . With the same collection data: 1Ƥ, 23, pinned ( LMCI 11-3-39 to 41), donated by the senior author to DZUP (DZ 22.349, 22.359 and 22.369); near 50 galls ( LMCI 11-3), 6 dissected adults ( LMCI 11-3-21) and 23 pupae ( LMCI 11-3-22), fixed and stored in 96% ethanol. Rincão da Ronda, Canguçu, RS, Brazil, 31°0 5’58’’S, 52°52’ 0 6’’W, October 2007, G.R.P.Moreira coll., 22 last instar larvae ( LMCI 6-1 to 15; 6-20; 6-21) and 8 pupae ( LMCI 6-18, 6-19), fixed and stored in 96% ethanol. With the same collection data, September 2007, 10 larvae ( LMCI 5-3), fixed and stored in 96% ethanol. Quebrada N. Estación Aforadora, Luján de Cuyo, Mendoza, Argentina, 32°53’40’’S, 69°13’49’’W, September 24, 2011, G. San Blas coll., 2 larvae and 1 pupa ( LMCI 163-13), dissected from galls on Schinus fasciculatus , fixed and stored in 96% ethanol.
Other cecidosid examined. Dicranoses congregatella: Rincão da Ronda, Canguçu, RS, Brazil, 31°05’58’’S, 52°52’06’’W, November 11, 2007, 3ƤƤ, 13, pinned ( LMCI 6-31 to 34), G.R.P.Moreira coll., reared from galls collected on Schinus polygamus . With the same collection data, July 20, 2007, several galls ( LMCI 3-13), 10 larvae (LMC 3-1 to 10), fixed and stored on ethanol 96%; September 9, 2007, 9 last instar larvae ( LMCI 5-4), 43 pupae ( LMCI 5-5; 5-6), fixed in Dietrich´s fluid and stored in ethanol 70%. Vila de Palmas, Bagé, RS, Brazil, 30°58’55’’ S, 53°37’10’’W, November 17, 2007, G.R.P. Moreira & G.L.Gonçalves colls., 16 pupae ( LMCI 12-33-1 to 16), dissected from galls collected from S. polygamus , fixed and stored in ethanol 96%. Av. Los Nogales y La Quinta, El Chorrillo Juana Koslay, San Luis, Argentina, 33°17’8’’ S, 66°15’32’W, October 10, 2009, G. San Blas coll., 4 larvae ( LMCI 163-7), dissected from galls on Schinus johnstonii , fixed and stored in 70% ethanol. Frente Reserva Florofaunística Merlo, San Luis, Argentina, 32°21’22’S, 64°57’33’W, October 21, 2009, G. San Blas coll., 6 pupae ( LMCI 163-11), dissected from galls on Schinus johnstonii fixed and stored in 70% ethanol. Cecidoses eremita: Rincão da Ronda, Canguçu, RS, Brazil, 31°05’58’’S, 52°52’06’’W, March 27, 2005, 2ƤƤ, 13, pinned ( LMCI 2-13 to 15), G.R.P.Moreira coll., reared from galls collected on Schinus polygamus (Cavanilles) Cabrera. With the same collection data, November 15, 2007, 22 last instar larvae (LMC 10-13-1 to 21). Morro Maximiano, Eldorado do Sul, RS, Brazil, 30°10’47’’S, 51°23’33’’W, March 13, 2007, G.R.P. Moreira & G.L.Gonçalves colls., 4 last instar larvae ( LMCI 16-44), 20 pupae ( LMCI 16-45), fixed in Dietrich´s fluid and stored in ethanol 70%. Potrerillos, Cerca Estación Aforadora, Luján de Cuyo, Mendoza, Argentina, 32°55’ 0 8’’S, 69°14’34’’W, November 14, 2009 ( LMCI 163-1; 2 larvae) and March 18, 2010 ( LMCI 163-3; 1 pupa), G. San Blas coll., dissected from galls on Schinus fasciculatus , fixed and stored in 96% ethanol. Eucecidoses sp.: Parque Passaúna, Campo Comprido, PR, Brazil, 25°27’42’’S, 49°22’54’’W, February 22, 2008, G.R.P. Moreira, O.S. Ribas, E. Carneiro & L. Beltrami colls., 34 last instar larvae ( LMCI 14-52; 14-53), dissected from galls collected on Schinus engleri , fixed in Dietrich´s fluid and stored in ethanol 70%. With the same collection data, March 20, 2010, G.R.P. Moreira & E. Carneiro, 11 dissected adults ( LMCI 80-75); near 80 galls, ( LMCI 80-76), fixed and stored in ethanol 96%. Eucecidoses minutanus: Las Compuertas, Las Heras , Mendoza, Argentina, 33°0 2’28’’S, 69°0 3’ 0 3’’W, October 26, 2011, G. San Blas & G.R.P. Moreira colls., 29 pupae ( LMCI 163-20 to 22), dissected from galls collected on S. fasciculatus , fixed and stored in 96% ethanol. Playa Hotel Villavicencio, Las Heras, Mendoza, Argentina, 32°31’39’’ S, 69°0 0’53’’W, May 1, 2011, G. San Blas coll., 4 larvae ( LMCI 163-4), dissected from galls collected on S. fasciculatus , fixed and stored in 70% ethanol. Cecidosidae sp. Tiltil, Rungue, Chile, 33°0 0’30’’S, 70°53’51’’ W, October 12, 2011, G. San Blas, G. Flores & R. Carrara colls., 3 larvae ( LMCI 163-14), dissected from galls collected on Schinus polygamus fixed and stored in 96% ethanol.
Diagnosis. Unlike adults of other Neotropical cecidosids, which have pale forewings and labial palpi either reduced or absent, those of O. argentinana have the body covered with uniform, shiny copper-colored scales and 2- segmented labial palpi, among other differences of genital structures. The pupa is characterized by possessing a cephalic, five-pointed frontal process (gall cutter), and a single large spine on the last abdominal tergum. Pupae of other Neotropical cecidosids have either single ( C. eremita and E. minutanus ) or three-pointed ( D. capsulifex and D. congregatela ) gall cutters. The larva is distinguished by having a uniform yellowish brown head, associated with a subretangular frontoclypeus that extends to the apex of the epicranial notch, and unpigmented ecdysial lines that delimit the posterior adfrontal enlarged areas, absent in the other Neotropical cecidosids. The gall is unique among cecidosids by being enclosed within swollen terminal branches of Schinus plants, without developing an external, fruit-like chamber that is characteristic for other gall-maker species.
Male adult ( Figs. 1 View FIGURE 1 A–4)
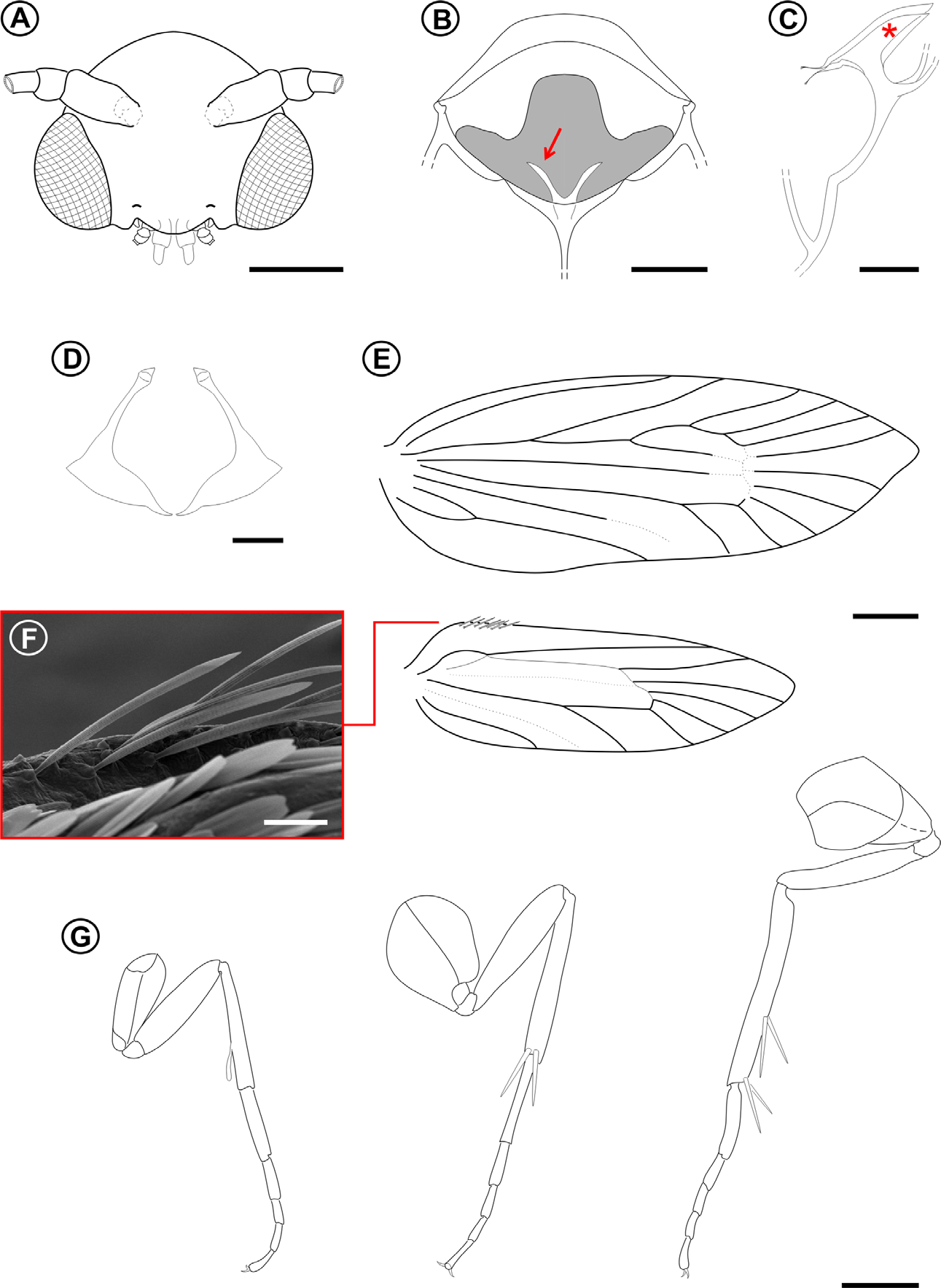
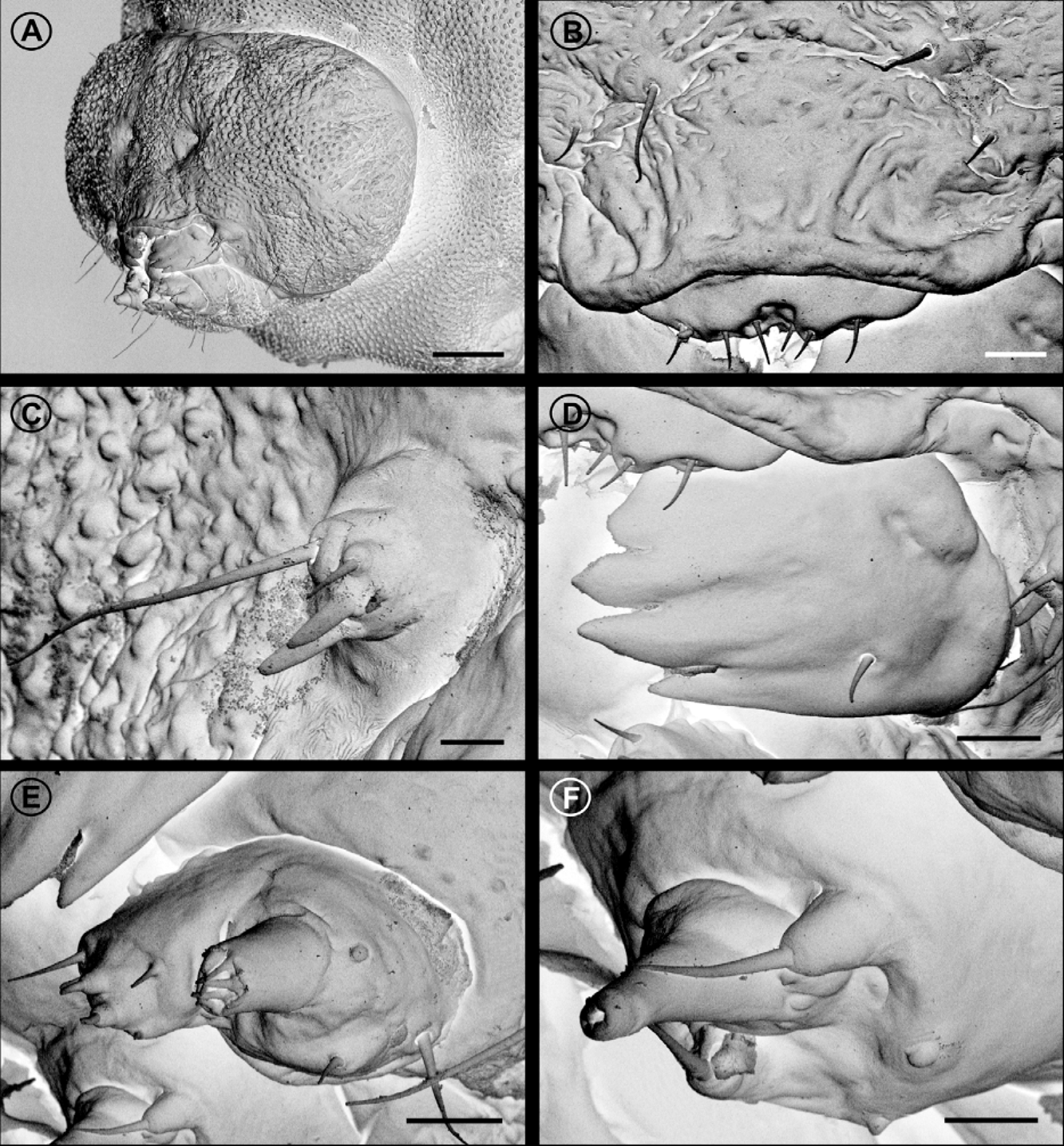
Forewing length 4.2 + 0.3 mm (n = 5).Wings, body, and other appendages uniformly of a shiny copper to reddish brown color ( Fig. 1 View FIGURE 1 ). Head: Frons and vertex smooth with sutures weakly developed ( Fig. 2 View FIGURE 2 A); vestiture consisting of a pair of latero-dorsal scale tufts curved forward over frons ( Fig. 3 View FIGURE 3 A). Frons covered by dense, piliform hairs ( Figs. 2 View FIGURE 2 A, B). Scales slender, lamellar, suberect, and scattered over labrum, maxillary, and labial palpi ( Fig. 3 View FIGURE 3 D). Eyes ( Figs. 2 View FIGURE 2 A, 3A) large (interocular index varying from 0.95 to 1.25; n = 4). Antennae short (varying from 0.52 to 0.56 length of forewing; n = 4); scape smooth except for medium dense pecten; flagellum filiform, with slender scales scattered only over dorsal half ( Fig. 3 View FIGURE 3 A); ventral half with elongate sensilla ca. 0.7x length of flagellomere ( Fig. 3 View FIGURE 3 C). Labrum greatly reduced ( Fig. 3 View FIGURE 3 D). Pilifers absent. Mandibles ( Fig. 3 View FIGURE 3 E) reduced to minute, sclerotized stubs. Haustellum ( Fig. 3 View FIGURE 3 G) reduced to minute lobes. Maxillary palpi ( Fig. 3 View FIGURE 3 G) 3-segmented, distal one reduced. Labial palpi ( Fig. 3 View FIGURE 3 F) 2-segmented, and short (circa 2/3 eye width in length).
Thorax: Anterior arms of laterocervical sclerites ( Fig. 2 View FIGURE 2 D) long and slender, with apex dilated. Metafurca ( Figs. 2 View FIGURE 2 B,C) with slender, elongate postero-dorsal apophyses free from secondary arms;, antero-dorsal apodemes absent. Wings ( Fig. 2 View FIGURE 2 E) lanceolate; microtrichia reduced in number; accessory cell present; retinaculum absent. Wing coupling ( Fig. 2 View FIGURE 2 F) consisting of ~15 frenular scales arising in two to three irregular rows near base of costa; Sc ending near midpoint of wing margin, radius with 5 free branches, M 3-branched, CuA 2-branched, CuA1 and M3 well separated from each other basally, CuP faint distally and not stalked with 1A+2A. Hindwing: ~ 2/3 forewing in length; Sc and R stalked and ending distally to midpoint of wing margin, Rs unbranched, M 3- branched, M1 and M2 well separated, CuA 2-branched, CuA1 and M3 well separated, CuP faded, not stalked with 1A+2A. Legs ( Figs. 2 View FIGURE 2 G) with spurs 0-2-4; epiphysis present. Tibial length proportion (anterior / medium / posterior legs) ~ 0.5/0.7/1.0.
Abdomen: Sternum 2 with broad, U-shaped caudal rim; tergosternal connection absent, as in C. eremita ( Davis 1998) .
Male genitalia ( Figs. 4 View FIGURE 4 A–C). Uncus shallowly bilobed. Socii consisting of a pair of oval setigerous lobes. Valva ( Fig. 4 View FIGURE 4 B) long and slender, with an elongate pectinifer along ventral margin extending ~ half length of valva. Vinculum Y-shaped. Aedeagus ( Fig. 4 View FIGURE 4 D), simple, slender and tubular, rosette-like shaped anteriorly; vesica without cornuti. Juxta ( Figs. 4 View FIGURE 4 C) elongate (~ 2/3 aedeagus length), slender, slightly spatulate distally, not divided but encircling aedeagus caudally. Saccus stout and tubular, with anterior apex slightly capitate.
Female adult ( Figs. 1 View FIGURE 1 B–4)
Similar to male; abdominal sternum7 with caudal margin rounded, as in C. eremita ( Davis 1998) .
Female genitalia ( Figs. 4 View FIGURE 4 E–G). Anterior apophyses long, extending beyond abdominal segment 5. Posterior apophyses fused distally. Apex of ovipositor ( Figs. 4 View FIGURE 4 F,G) compressed and sagittate; ventral ridge with minute serrations. Cloaca with two apodemes that extend beyond abdominal segment 7. Spermatheca without lateral lagena; caudal part of ductus spermatheca not coiled. Vestibulum without sclerotized structures; ductus and corpus bursae membranous; signum absent.
Immature stages
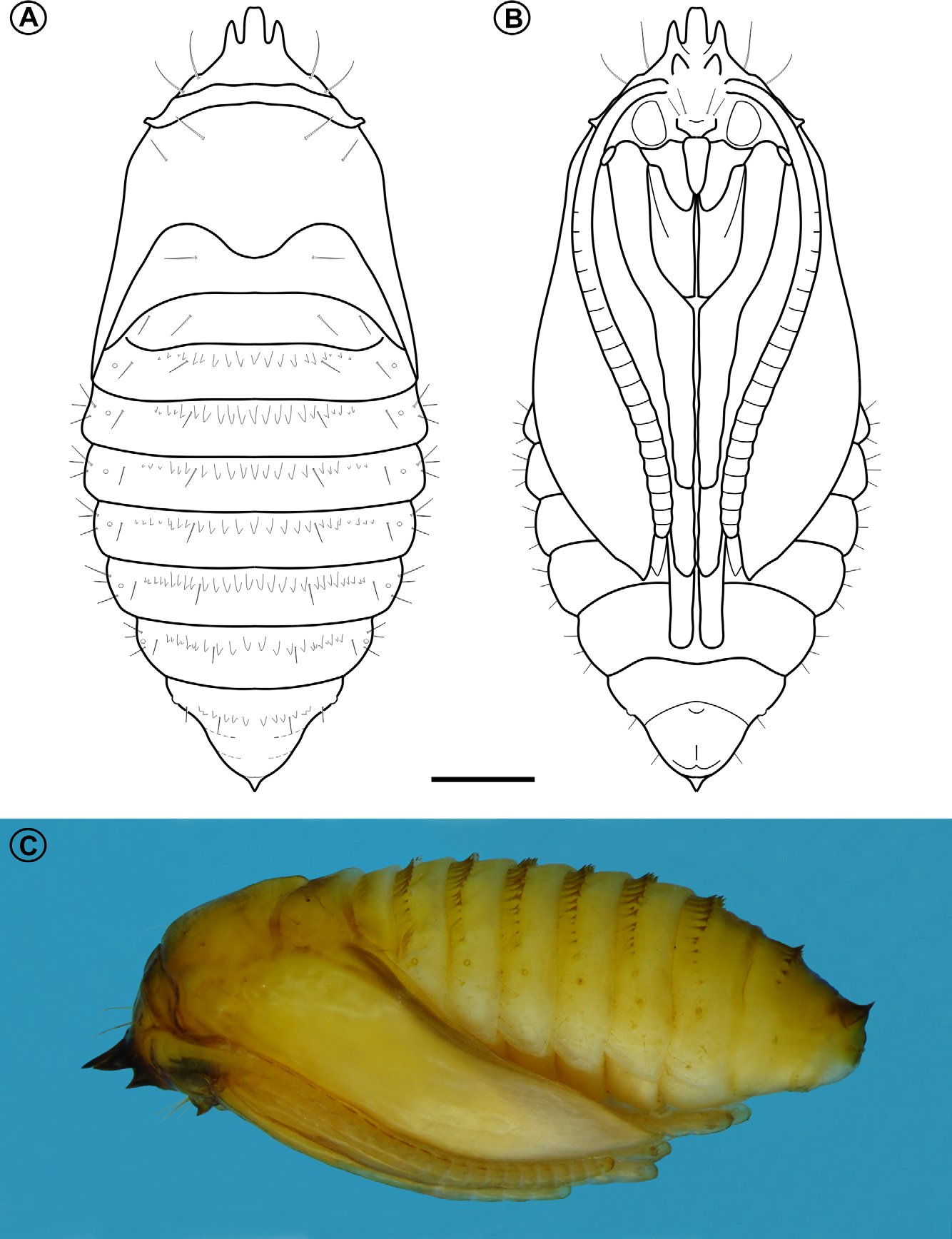
Last instar larva ( Figs. 5–7 View FIGURE 5. O View FIGURE 6 View FIGURE 7 ). Body length = 3.2+0.17 mm; head capsule width = 0.7+0.8 mm; n = 5. Prognathous, with chewing mouthparts; body cylindrical, covered with microtrichia; primary setae reduced.
Head dorsal (5B–C, 6A) yellowish brown, ~ 2x broader than high, with convex lateral margin; frontoclypeus well marked by pigmented adfontral sutures ( Fig. 5 View FIGURE 5. O B), subretangular in shape posteriorly, extending to apex of epicranial notch; ecdysial lines unpigmented, delimiting two semicircular, posteriorly located adfrontal areas. Head ventral ( Fig. 5 View FIGURE 5. O C) with well marked, slightly divergent hypostomal ridges; hypostomal lobes subtriangular; basistipes and postmentum areas unpigmented. Stemmata absent; antennae ( Fig. 6 View FIGURE 6 C) reduced; labrum ( Fig. 6 View FIGURE 6 B) bilobed, with three pairs of small setae on distal margin; mandible ( Fig. 6 View FIGURE 6 D) well developed with four cusps along distal margin and one small seta basally on external surface; maxilla ( Fig. 6 View FIGURE 6 E) with palpus and galea poorly developed; spinneret ( Fig. 6 View FIGURE 6 F) tubular, equal in length to maxillary palpus; labial palpus ( Fig. 6 View FIGURE 6 F) one-segmented, with well developed apical seta. Chaetotaxy consisting of 13 pairs of setae: F group unisetose; C group bisetose; AF group bisetose; A group trisetose; P group unisetose; L group unisetose; S group trisetose.
Thorax and abdomen ( Figs. 5 View FIGURE 5. O , 7 View FIGURE 7 ) white; prothoracic shield ( Figs. 5 View FIGURE 5. O B) consisting of pair of irregularly shaped, yellowish brown areas; A1–7 with well developed calli, centrally on posterior margin of terga; thoracic legs reduced to circular, unsegmented tubercles ( Fig. 7 View FIGURE 7 C, D); prolegs absent; circular spiracles without elevated peritreme ( Fig. 7 View FIGURE 7 E) laterally on T1, A1–8. Abdominal segment 10 ( Fig. 7 View FIGURE 7 F) composed of three lobes, one dorsal and two lateral. Thoracic and abdominal setae reduced in number and size. T1 with 7 pairs of setae; SD group bisetose; L group bisetose; V group bisetose. T2–3 with 4 pairs of setae; SD group unisetose; SV unisetose; V group bisetose. A1–9 with 2 pairs of setae; SD and L, unisetoses. A10 with 4 pairs of setae; SD unisetose; SV bisetose; V unisetose.
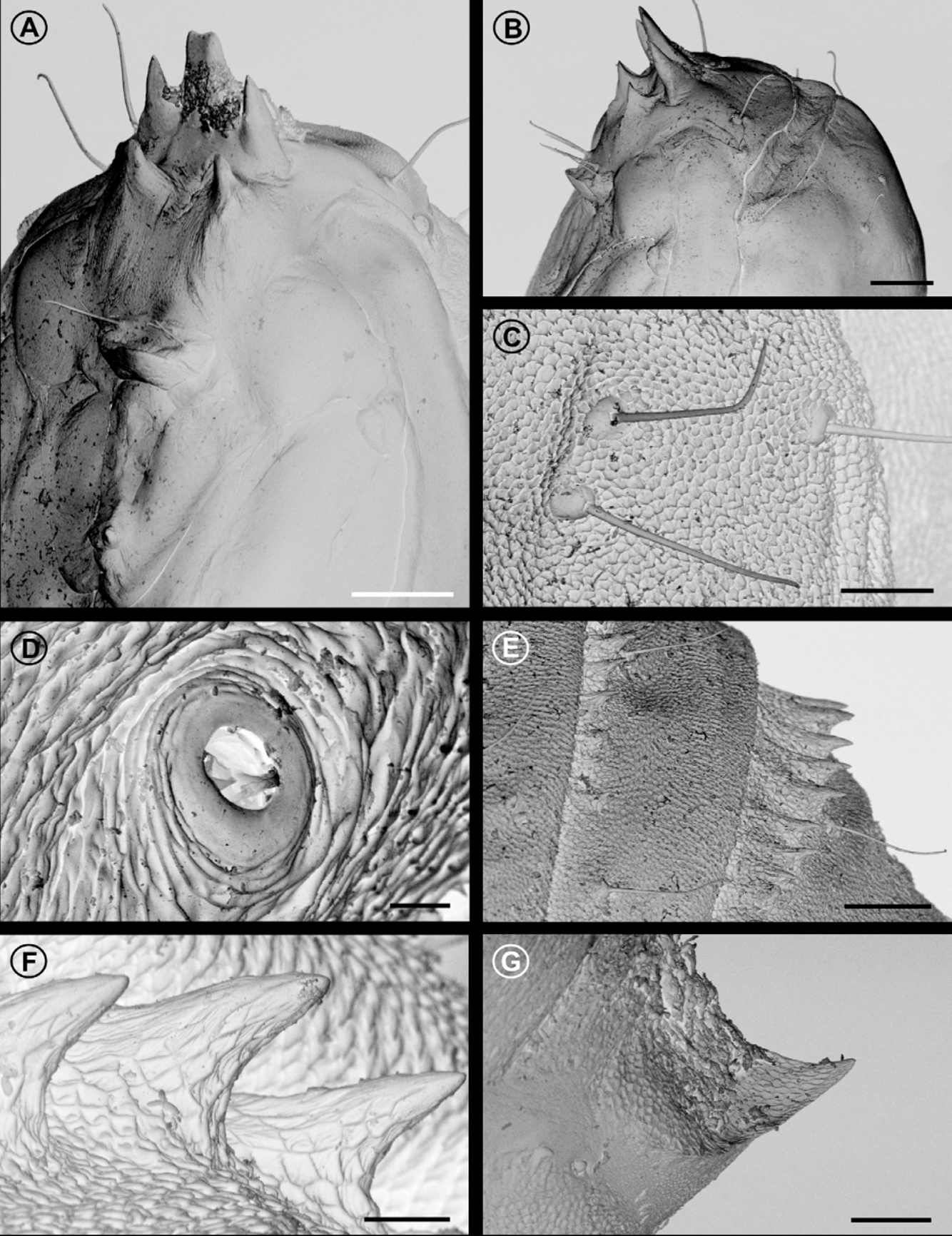
Pupa ( Figs. 8 View FIGURE 8. O , 9 View FIGURE 9. O ). Length = 3.723+ 0.102 mm; n = 6. Yellowish brown, becoming dark brown near adult emergence ( Fig. 10 View FIGURE 10. O F); head with frontal processes ( Figs. 9 View FIGURE 9. O A,B) used for cutting the gall chamber wall, formed by five large spines, grouped into two parallel rows: dorsalmost row with three apical processes, middle process blunt, lateral ones acute, ventralmost row with two smaller, pointed processes; frons and lateral portion of vertex with two pairs of setae each; antennae narrow, long, apex near forewing apex; prothorax a narrow transverse band between head and mesothorax; hindwings concealed by forewings, reaching sternum A6; metathoracic legs reaching beyond forewing apex, on segment A7; tergaT2–3 with a pair of latero-dorsal setae. Abdominal segments covered by microtrichia; A2–8 with a transverse band of spines ( Figs. 9 View FIGURE 9. O E, F), near anterior margin of terga; tergum A10 with anteriorly directed, acute process on posterior margin ( Fig. 9 View FIGURE 9. O G). Setae arranged in three rows (dorsal, supra- and subspiracular); one dorsal pair on segments A1–8; one supra-spiracular pair on segments A2–8; three subspiracular pairs on segments A3–7, one pair on A8–9; spiracle circular ( Fig. 9 View FIGURE 9. O D), without elevated peretreme laterally on A2–8, spiracle on A8 greatly reduced.
Gall ( Fig. 10 View FIGURE 10. O ). Spindle-shaped, enclosed within swollen stems ( Figs. 10 View FIGURE 10. O A, B) on the host plant terminal branches. Larval chamber elliptical in shape (maximum diameter = 3.8+0.15mm; n = 6), transversally located in relation to the stem axis ( Figs. 10 View FIGURE 10. O C–E); with an external shallow wall, formed as an expansion of the wood tissue, under the bark. Unlike other Neotropical cecidosids, an operculum is lacking. With the advent of pupation, a progressive necrosis and eventual death of the external bark tissue of the gall occur which results in a thinning of the outer wall of the chamber ( Fig. 10 View FIGURE 10. O F). With the action of the frontal process and body contortions, the pupa opens an irregular, lateral orifice ( Fig. 10 View FIGURE 10. O G). By continuing these movements and anchoring the body laterally with its abdominal spines, the pupa pushes itself partially out of the gall. During this process, the anterior portion of the exuvia is split, allowing adult emergence. In most cases after adult emergence, the anterior part of the pupal exuvium (head and thorax) is found protruding to the outside, while the posterior third remains in the chamber ( Fig. 10 View FIGURE 10. O H). After emergence, the outer wall of the chamber eventually collapses, with the empty galls appearing as small craters on the host plant stem surface ( Fig. 10 View FIGURE 10. O I).
Host plant. In Rio Grande do Sul (RS) state, Brazil, O. argentinana galls have been found on plants identified in most Brazilian herbaria as Schinus polygamus (sensu Cabrera 1938; Fleig 1987, 1989). The identity of such plants should be taken with caution because the taxonomy of the South American species of Schinus is controversial and in need of revision (for a discussion, see Barkley 1957, Burckardt & Basset 2000, and Steibel & Troiani 2008). According to Barkley (1957), records of S. polygamus from Uruguay might pertain to S. fasciculatus , which may also be true for those from the southern part of RS. According to that author, the distribution of S. polygamus is restricted to Chile. In Mendoza, Argentina, such galls are in fact found on S. fasciculatus , as already mentioned.
Distribution. In general, Neotropical cecidosids are rarely represented in insect collections. Oliera argentinana was originally described from material collected in the Buenos Aires province, Argentina ( Brèthes 1916). The species was recently collected further west in the Argentinean province of Mendoza, where apparently it is rare. It has been found further east by the first author in the Southeastern Highlands of Rio Grande do Sul state, Brazil. It was not listed by Biezanko et al. (1957) from Uruguay, possibly due to inadequate collecting. Thus, the meager distributional data available suggest that O. argentinana occurs primarily within the Pampa province of the Chacoan subregion (sensus Morrone 2006).
Life history. Little is known about the biology of O. argentinana . In southern Brazil, their galls have been collected occasionally, and concentrated primarily on a few hosts among S. polygamus plants within a given locality. They may, however, occur in dense concentrations on single plants and share the same host with other cecidosids, such as C. eremita and D. congregatella . In agreement with Brèthes (1916), our field collection dates indicate that the species is univoltine in Argentina and Southern Brazil, with adult emergence occurring from late Spring to early Summer (November / December).
Molecular phylogeny. A total of 626 nucleotide sites were analyzed, in which 262 sites were variable and 230 parsimony informative. Genetic divergence among major lineages tested, including cecidosid genera and outgroups, varied from 15 to 28% ( Table 2 View TABLE 2 ). Distance, ML and MP analyses showed identical topology with different bootstrap supports ( Fig. 11 View FIGURE 11 ). Oliera argentinana was strongly supported as a monophyletic clade positioned within Cecidosidae and related to Eucecidoses . The node that joins Oliera + Eucecidoses was recovered either in distance, probabilistic or parsimony analyses, with bootstrap support higher than 60%. The clade Oliera + Eucecidoses is likely the sister group of Cecidoses . These three clades together represent the current status of Neotropical Cecidosidae (excluding Dicranoses ). In addition, the resulting phylogeny indicated that Dicranoses is not closely related to the other cecidosid genera, because the Prodoxidae and Adelidae examined were positioned between these groups, thus leaving Dicranoses as an outgroup ( Fig.11 View FIGURE 11 ).
Results found in the present study have relevant implications not only for the taxonomy and phylogeny of O. argentinana but also for the other supra-specific lineages of Neotropical Cecidosidae . They also suggest the existence of a greater diversity for the family in southern South America, which should be explored further.
In the original work of Brèthes (1916), the external morphology of the gall, larva, pupa and adult stage of O. argentinana were illustrated. Although lacking details, his general description gave three conspicuous characters that clearly separate this species from other described South American cecidosids: 1) gall formation restricted to under bark of terminal branches, without developing an external, fruit-like chamber; 2) pupa with a cephalic frontal process (gall cutter) formed by five large spines, grouped into two rows, with three spines in the anterior and two in the posterior row; 3) adult uniformly covered by reddish brown scales. Because the specimens we examined agree completely with these features, we conclude that the original description of the gall of O. argentinana presented by Brèthes (1916) is accurate. We complement it further with data from scanning electron microscopy and DNA sequences. The lectotype of O. argentinana is in poor condition; it is basically only a thorax on a pin with a slide of the damaged genitalia. But it shows clear diagnostic similarities, as for example the stout saccus and enlarged, semicircular anterior end of the aedeagus, which are most characteristic for O. argentinana .
The redescription presented by Parra (1998) of what was allegedly C. argentinana from Chile diverges markedly from the features presented above for Argentinean material. Parra described the gall as a chamber on a short stalk protruding externally from the plant stem, the frontal process of the pupa as truncated and conical, and the adult colour as varying from greyish to whitish-grey with scattered black scales on the forewings. In addition, the larva studied by Parra (1998) possessed two stemmata, structures that are absent in all known Neotropical Cecidosidae . Also, the pupa illustrated by him possessed conspicuous abdominal spines whose size, shape and arrangement are different from those known for the remaining species. Furthermore, the adult figured by Davis (1999), as O. argentinana , illustrated from material collected in Chile and supposedly conspecific with that examined by Parra (1998), has a 3-segmented labial palpi, contrary to what we described herein. The DNA analysis in this study included specimens from Chile (treated here as Cecidosidae sp.), which morphologically are similar to the material studied by Parra (1998). Our results give further support to our hypothesis that Parra misidentified the species he studied, because such specimens arose not within the Oliera clade, but as a sister group of the Eucecidoses + ( Oliera + Eucecidoses ) clade ( Fig.11 View FIGURE 11 ). We conclude that the specimens studied by Parra (1998) and Davis (1999) belong to either one or two different cecidosid lineages, which will be treated elsewhere (San Blas, G. et al. in prep.).
Systematic position of Oliera . Results from both morphology and DNA do not support the proposition by Parra (1998) that Oliera is synonymous to Cecidoses . Instead, we found Oliera to be more closely related to Eucecidoses . Other species should be assigned to this genus with increased collecting efforts for Neotropical cecidosids. According to preliminary molecular results, not included in the present study, there are at least two additional congeneric species to O. argentinana , which are known only from the larval stage, collected recently in Chile and Brazil (San Blas, G. & Moreira, G.R.P., unpubl. data). Furthermore, our observations revealed that these genera present major, consistent morphological differences for most life stages (larva, pupa and adult). Oliera argentinana specimens have the following characteristics, which are not found on the other Neotropical cecidosids examined: 1) gall developing under the bark of terminal branches, without developing an external, fruit-like chamber; 2) larva with pronounced, posteriorly rectangular frontoclypeus with apex extended to the epicranial notch, associated with unpigmented ecdisial lines that delimit two semicircular, posteriorly located adfrontal areas; 3) pupa with cephalic frontal process composed of 5 separate individual processes, and last abdominal segment with a single, dorsal acute spine; and, 4) adult with 2-segmented labial palpi.
Phylogeny and systematic of Cecidosidae . Our study shows that genetic divergence among Neotropical cecidosid genera (> 20% in most cases) is nearly equal or greater than the divergence between them and the related adeloid families Prodoxidae and Adelidae , herein used as outgroups. Such results do not support the proposition of Becker (1977) regarding the synonymy of Eucecidoses with Cecidoses . Thus, we propose that Eucecidoses be reinstated as valid. We are in the process of revising Eucecidoses . Our preliminary results indicate a greater diversity of Eucecidoses species in southern Brazil than previously suspected.
Finally, our molecular analysis suggests that the family Cecidosidae (sensu Nielsen & Davis 1985; Davis 1999) may be paraphyletic. Dicranoses congregatella came out as the sister group of a cluster comprising species belonging to the Adelidae (Adela) and the Prodoxidae ( Tetragma + Greya + Prodoxus ), and not closely related to the clade of Cecidoses + ( Eucecidoses + Oliera ) as was initially expected. In addition, distance-based analysis revealed pairwise divergences among cecidosid lineages to be as high as those between Cecidosidae and the outgroups. The Prodoxidae is considered to be the sister group of Cecidosidae (Pellmyr & Leebens-Mack 1999; Hoare & Dugdale 2003). We plan to obtain additional DNA sequences, using more conserved gene markers and also to study Dicranoses capsulifex Kieffer & Jörgensen, 1916 , the type species of Dicranoses , in order to test further the composition and phylogenetic relationships of the Cecidosidae .
TABLE 2. Estimates of evolutionary divergence between sequences based on 626 base pairs of cytochrome oxidase I (COI) gene using Kimura 2 - parameter model (Kimura 1980). Average number (± standard error) of base substitutions per site over all sequence pairs between groups, obtained by a bootstrap procedure of 1000 replicates are shown. The analysis involved 24 specimens belonging to five Neotropical cecidosid lineages and two outgroups (Prodoxidae and Adelidae).
| Oliera | Dicranoses | Cecidosidae sp. | Cecidoses | Eucecidoses Prodoxidae | |
|---|---|---|---|---|---|
| Oliera | - | ||||
| Dicranoses | 0.26 ± 0.03 | - | |||
| Cecidosidae sp. | 0.15 ± 0.02 | 0.25 ± 0.03 | - | ||
| Cecidoses | 0.18 ± 0.02 | 0.28 ± 0.03 | 0.21 ± 0.03 | - | |
| Eucecidoses | 0.17 ± 0.02 | 0.25 ± 0.03 | 0.18 ± 0.03 | 0.21 ± 0.03 | - |
| Prodoxidae | 0.25 ± 0.03 | 0.28 ± 0.03 | 0.25 ± 0.03 | 0.24 ± 0.03 | 0.25 ± 0.03 - |
| Adelidae | 0.22 ± 0.03 | 0.24 ± 0.03 | 0.26 ± 0.03 | 0.22 ± 0.03 | 0.26 ± 0.03 0.18 ± 0.03 |
| Discussion |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |