Nymphaea subg. Hydrocallis
|
publication ID |
https://doi.org/10.1016/j.phytochem.2014.04.007 |
|
DOI |
https://doi.org/10.5281/zenodo.10561429 |
|
persistent identifier |
https://treatment.plazi.org/id/03BA87CE-FFBB-101C-FCA9-F6287FDDF836 |
|
treatment provided by |
Felipe |
|
scientific name |
Nymphaea subg. Hydrocallis |
| status |
|
2.1. Floral scent composition of Nymphaea subg. Hydrocallis
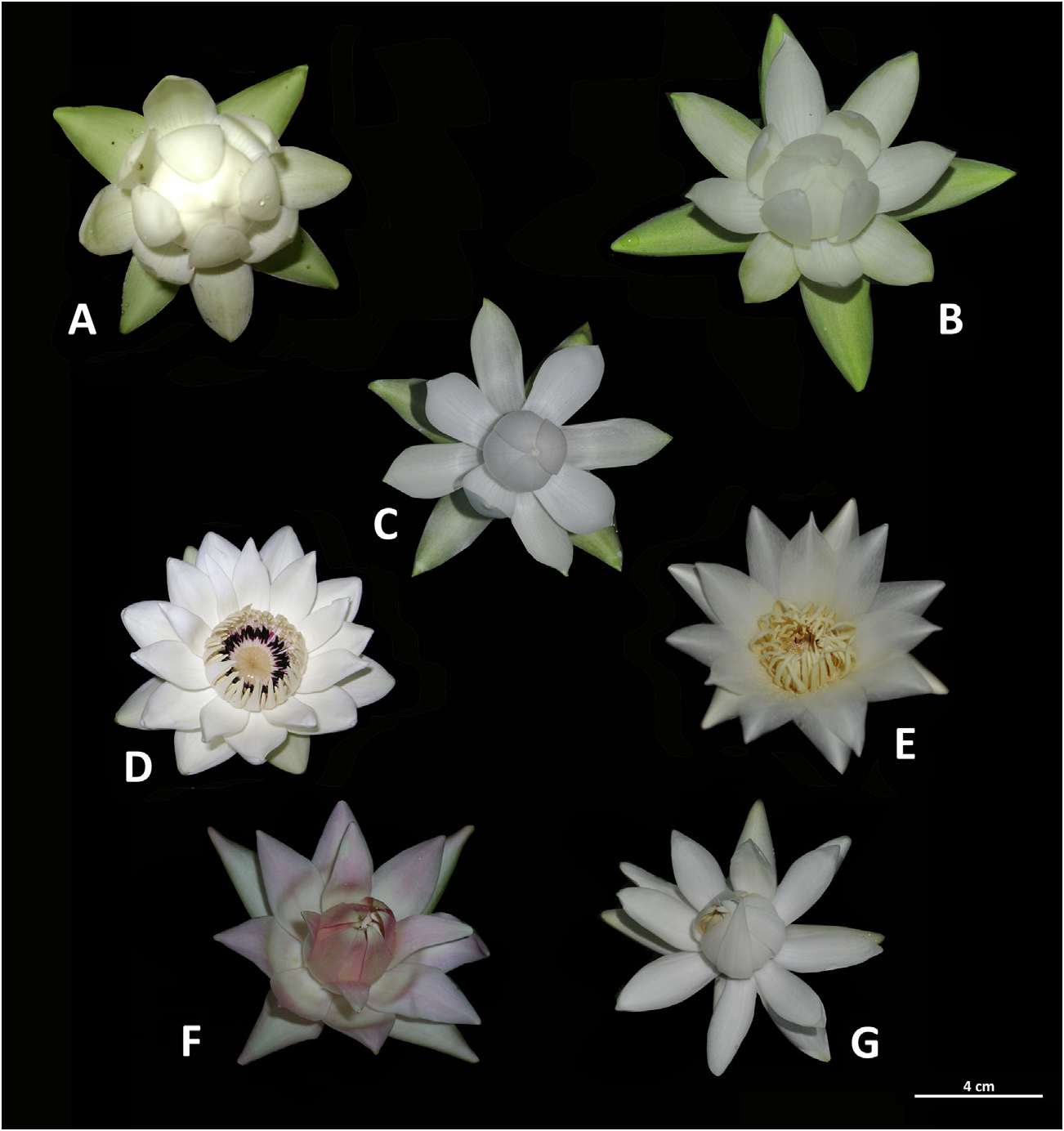
The six species and two subspecies of Nymphaea subg. Hydrocallis investigated are all native to Brazil and three of them are known to be associated with pollinator cyclocephaline scarabs ( Fig. 1 View Fig ; Table 1 View Table 1 ).
To the human nose, flowers of the seven investigated taxa of Nymphaea subg. Hydrocallis were remarkably fragrant during the consecutive evenings of the pistillate ($) and staminate (#) phases of anthesis. While the flowers of N. rudgeana and N. gardneriana emitted a pungent, fermented fruity odor with solvent-like reminiscents, the scents of the remaining species all bore a strong, uncharacteristic solvent-like odor ( Table 1 View Table 1 ).
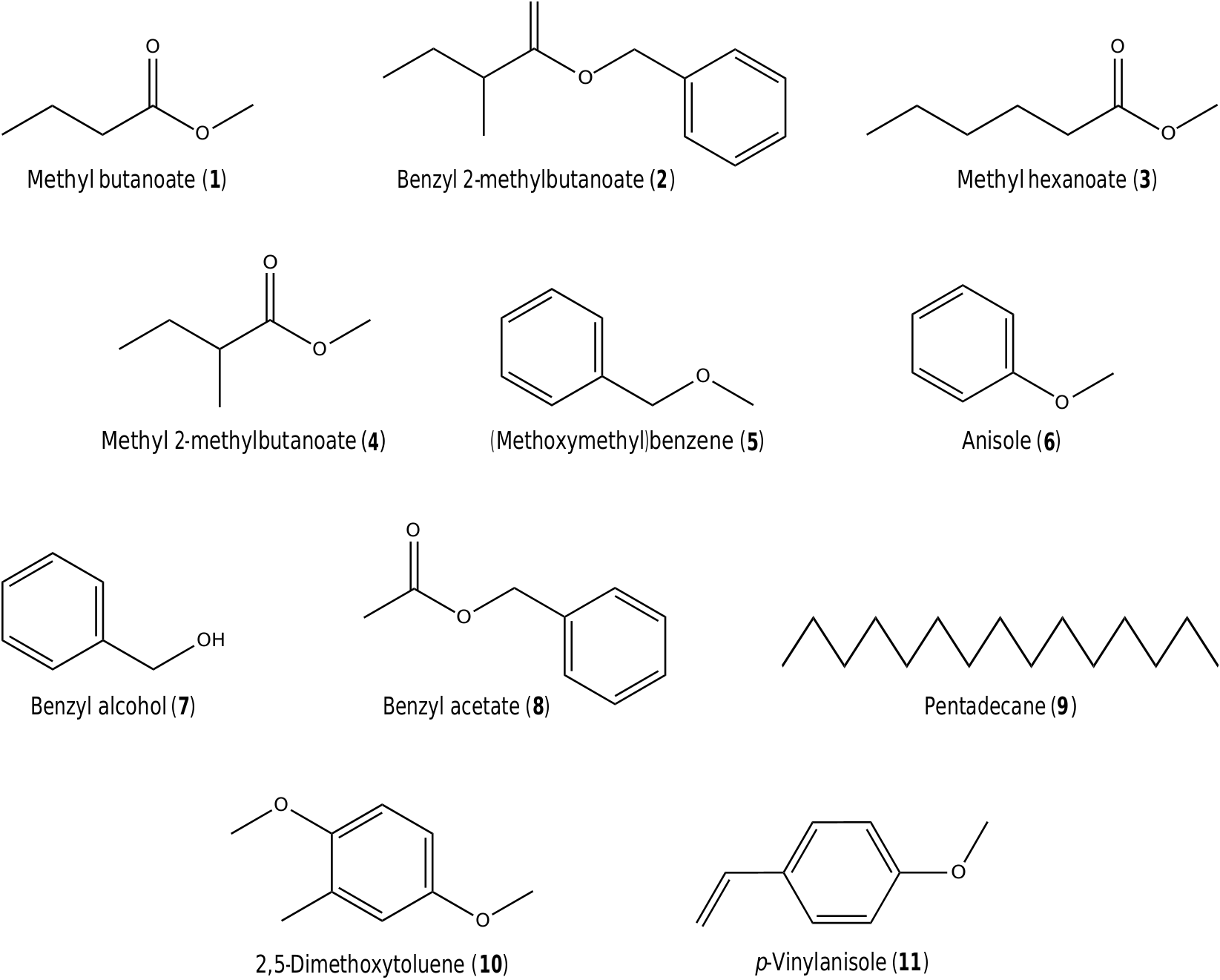
The chemical analysis showed that the seven studied taxa emitted floral volatiles in different quantities and compositions. A total of 22 compounds were identified in the analyzed samples, ranging in molecular weight from 102 [methyl butanoate] ( 1) to 192 [benzyl 2-methylbutanoate] ( 2) ( Fig. 2 View Fig ; Table 2 View Table 2 ). The identified volatile compounds belong to three of the seven compound classes proposed by Knudsen et al. (2006): aliphatics (9), C5-branched chain compounds (5) and aromatics (8).
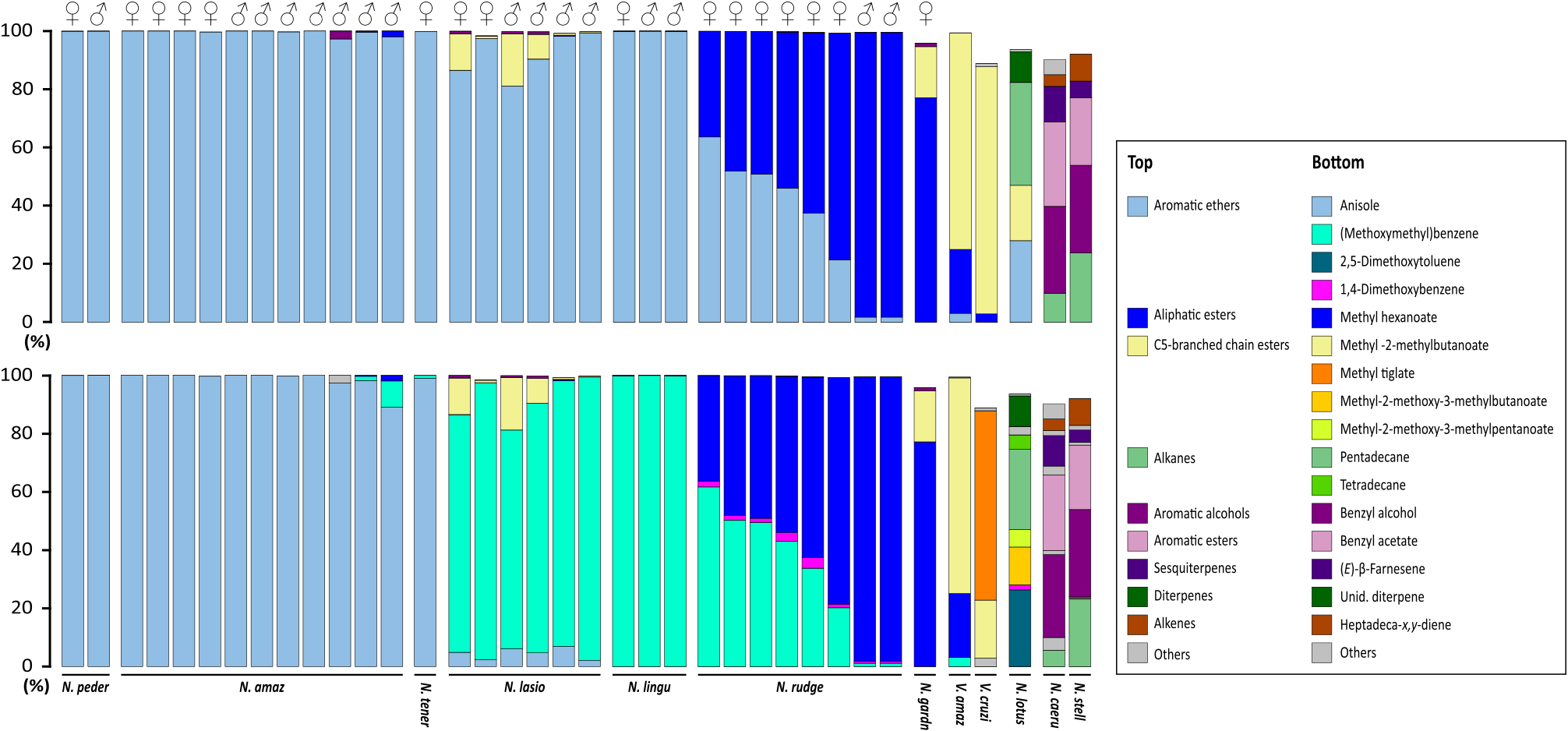
The number of compounds per species ranged from only two in N. lingulata , N. amazonum and N. tenerinervia , to 12 in N. lasiophylla ( Table 2 View Table 2 ). Dominant compounds reaching an overall relative percentage content of at least 10% in the analyzed scent samples were methyl hexanoate ( 3) in N. rudgeana (36.3–77.8% $; 81.0–97.7% #) and N. gardneriana (21.9% $); methyl 2-methylbutanoate ( 4) in N. lasiophylla (0.8–12.5% $; 0.4–18.0% #) and N. gardneriana (74.0% $); (methoxymethyl)benzene ( 5) in N. rudgeana (20.2–61.7% $; 1.2–15.7% #), N. lasiophylla (81.6–95.1% $; 75.1–97.2% #) and N. lingulata (99.8% $; 99.8–99.9% #); and anisole ( 6) in N. tenerinervia (98.9% $), N. amazonum subsp. amazonum (99.7–100.0% $; 89.0– 100.0% #) and N. amazonum subsp. pedersenii (99.9% $; 99.9% #) ( Figs. 2 View Fig and 3 View Fig ; Table 2 View Table 2 ).
No marked differences in floral scent composition between pistillate and staminate phases of anthesis could be evidenced, with the sole exception of the analyzed samples of N. rudgeana ($, n = 4; #, n = 2), in which the emission of (methoxymethyl)benzene ( 5) was dramatically reduced during the staminate phase ( Fig. 3 View Fig ; Table 2 View Table 2 ).
The rates of floral scent emission differed between species (PERMANOVA: df = 6, F = 46.709, R 2 = 0.9, P = 0.031) and phases of anthesis (PERMANOVA: df = 1, F = 5.043, R 2 = 0.016, P = 0.031). The interaction between these two factors had no significant effect (PERMANOVA: df = 4, F = 1.477, R 2 = 0.019, P = 0.262), meaning that variations of scent between the pistillate and the staminate phases showed the same pattern for each species: floral scent discharge was higher at the pistillate phase (865 ± 194 µg h – 1, n = 16) than at the staminate phase (392 ± 97 µg h – 1, n = 16).
Among the different species from subg. Hydrocallis , only N. amazonum subsp. amazonum (144 ± 29 µg h – 1, n = 11), N. lasiophylla (436 ± 54 µg h – 1, n = 6), and N. rudgeana (1654 ± 138 µg h – 1, n = 8) exhibited significantly different rates of floral scent emission. N. lingulata (497 ± 99 µg h – 1, n = 3) and N. amazonum subsp. pedersenii (389 ± 56 µg h – 1, n = 2) did not show any significant difference in floral scent emission rates when compared to any of the other sampled species, and only a single sample of N. tenerinervia (121 µg h – 1) and N. gardneriana (275 µg h – 1) each were analyzed.
Table 2 Chemical composition (amounts of each compound) of the floral scent of seven species of Nymphaea subg. Hydrocallis (Nymphaeaceae). Floral scent samples were obtained by perceivable odor emission in the course of the pistillate (day 1; $) and staminate (day 2; #) phases of anthesis.
| Species list | RI | N. rudge | N. lasio | N. lingu | N. amaz | N. peder | N. gard | N. tener | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| $ ( n = 6) | # ( n = 2) | $ ( n = 2) | # ( n = 4) | $ ( n = 1) | # ( n = 2) | $ ( n = 4) | # ( n = 7) | $ ( n = 1) | # ( n = 1) | # ( n = 1) | # ( n = 1) | ||
| Total number of compounds | 8 | 7 | 8 | 11 | 2 | 2 | 1 | 3 | 3 | 2 | 11 | 3 | |
| Total amount of scent per flower (µg h – 1) | 1368–2123 | 973–1554 | 328–651 | 270–475 | 651 | 313–528 | 56–330 | 43–278 | 445 | 333 | 275 | 121 | |
| Aliphatics | |||||||||||||
| Esters | |||||||||||||
| Methyl butanoate | <800 | – | – | – | – | – | – | – | – | – | – | 0.53 | – |
| Butyl acetate | 815 | – | – | – | – | – | – | – | – | 0.04 | – | – | 0.05 |
| Methyl hexanoate | 928 | 36.28–77.78 | 81.03–97.69 | 0–0.05 a | 0–0.42b | – | – | – | 0–1.96b | – | – | 21.94 | – |
| Methyl pentanoate | 824 | – | – | – | – | – | – | – | – | – | – | 0.02 | – |
| ( Z)-methyl hex-3-enoate | 933 | – | – | – | – | – | – | – | – | – | – | 0.05 | – |
| ( E)-methyl hex-3-enoate | 937 | – | – | – | – | – | – | – | – | – | – | tr | – |
| ( E)-methyl hex-2-enoate | 966 | 0.03–0.47 | 0.10–0.20 | – | – | – | – | – | – | – | – | 0.03 | – |
| Methyl heptanoate | 1026 | 0.01–0.08 | 0.03–0.08 | – | – | – | – | – | – | – | – | 0.02 | – |
| Methyl octanoate | 1126 | 0.03–0.25 | 0.11–0.21 | – | – | – | – | – | – | – | – | – | – |
| C5-branched chain compounds | |||||||||||||
| Esters | |||||||||||||
| Methyl 2-methylbutanoate | <800 | – | – | 0.83–12.54 | 0.36–17.96 | – | – | – | – | – | – | 74.02 | – |
| Ethyl 2-methylbutanoate | 849 | – | – | – | 0–0.21a | – | – | – | – | – | – | – | – |
| Methyl 2-hydroxy-2-methylbutanoate | 849 | – | – | – | 0–0.04b | – | – | – | – | – | – | – | – |
| Methyl tiglate | 865 | – | – | – | – | – | – | – | – | – | – | 0.28 | – |
| Methyl 3-hydroxy-2-methylpropanoate | 893 | – | – | 0–0.02 a | 0–0.07b | – | – | – | – | – | – | – | – |
| Aromatics | |||||||||||||
| Alcohols | |||||||||||||
| Benzyl alcohol | 1033 | 0–0.02 c | – | 0.09–0.88 | 0–0.87b | 0.22 | 0.09–0.25 | – | – | – | – | – | – |
| 2-(4-methoxyphenyl)ethanol | 1244 | 0–0.16d | 0.05–0.18 | – | – | – | – | – | 0–2.72b | – | – | – | – |
| Aldehyde | |||||||||||||
| Benzaldehyde | 959 | – | – | – | 0–0.10b | – | – | – | – | – | – | – | – |
| Esters | |||||||||||||
| Methyl benzoate | 1095 | – | – | 0–0.04 a | 0–0.05b | – | – | – | – | – | – | – | – |
| Benzyl 2-methylbutanoate | 1388 | – | – | – | 0–0.01 a | – | – | – | – | – | – | – | – |
| Ethers | |||||||||||||
| Anisole | 917 | – | – | 2.31–4.88 | 2.15–6.89 | – | – | 99.66–100.00 | 88.98–100.00 | 99.91 | 99.94 | 98.94 | |
| (methoxymethyl)benzene | 988 | 20.20–61.66 | 1.16–15.69 | 81.57–95.12 | 75.10–97.24 | 99.78 | 99.75–99.91 | – | 0–9.06b | 0.01 | 0.02 | 3.10 | 1.00 |
| 1.4-dimethoxybenzene | 1164 | 1.23–3.65 | 0.61–2.85 | 0–0.01 a | |||||||||
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |