Myotrioza oppositifoliae Taylor
|
publication ID |
https://doi.org/10.11646/zootaxa.4073.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:A502D3A2-C070-4E9D-9F55-BA07C731FCF3 |
|
DOI |
https://doi.org/10.5281/zenodo.6063730 |
|
persistent identifier |
https://treatment.plazi.org/id/03FA87E9-E542-FFEB-6ED5-BB20FD756B58 |
|
treatment provided by |
Plazi |
|
scientific name |
Myotrioza oppositifoliae Taylor |
| status |
sp. nov. |
Myotrioza oppositifoliae Taylor View in CoL , sp. nov.
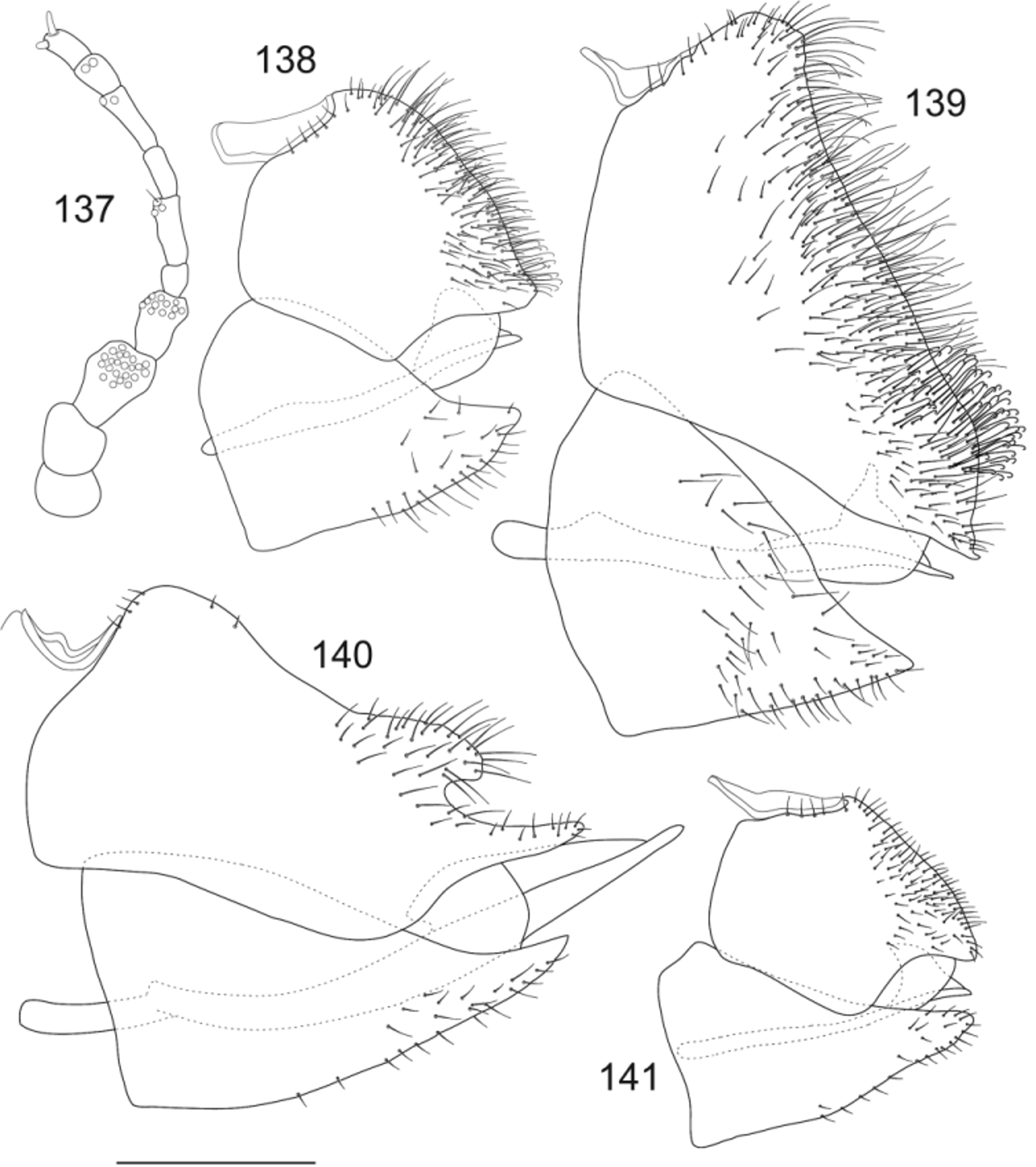
( Figs 140 View FIGURES 137 – 141 , 150–157 View FIGURES 150 – 157 , 176–177 View FIGURES 174 – 181 , 183 View FIGURES 182 – 185 ; Tables 1–8 View TABLE 1 )
Types. AUSTRALIA, Western Australia: Holotype: 1 ♂ (slide) Credo Station Reserve, Coolgardie North Rd, 30º25.402'S, 120º48.242'E, G.S. Taylor, 3.ix.2011, swept Eremophila oppositifolia, 2011 148, CR29 (WAM). Paratypes: 1 ♀ (dried), 2 ♀ (slide), same data as holotype (WAM, WINC). Other specimens examined. Western Australia: 2 ♂ (ethanol) Credo Station Reserve, Coolgardie North Rd, 30º13.306'S, 120º38.520'E, G.S. Taylor, 5.ix.2011, swept Eremophila sp. (salmon-yellow flowers), 2011 166, CR47 (WAM).
Description. Adult ( Figs 150–153 View FIGURES 150 – 157 ). Colouration. Male: Pale yellow brown: vertex with indistinct pale orange brown marking in vicinity of fovea; eyes greyish brown; antennal segments 8–10 progressively dark brown; mesopraescutum with a pair of pale orange brown anterior submedial markings; mesoscutum with two pairs of pale orange brown submedial markings; fore wings with brown infuscation; hind wings clear; fore wing veins equally pigmented brown; legs pale yellow-brown; abdominal tergites 1–5 with greyish infuscation; abdominal membrane colouration pale green; proctiger, subgenital plate and parameres yellow-brown; basal portion of proctiger and apices of parameres black. Female: as for male except head and thorax with pale green suffusion; abdominal membrane colouration darker green; abdominal tergites with brown lateral marking; sternites with brown lateral infuscation; proctiger and subgenital plate pale yellow-brown with green infuscation with apices strongly sclerotised, dark brown to black.
Structure. Measurements as in Tables 4–8 View TABLE 4 View TABLE 5 View TABLE 6 View TABLE 7 View TABLE 8 . Body short, compact ( Figs 150–153 View FIGURES 150 – 157 ). Head ( Figs 154–155 View FIGURES 150 – 157 ); vertex with weak medial suture, sunk in vicinity of fovea; genal processes short, 0.37–0.48 times as long as vertex; antenna very short, 0.63–0.73 times width of head, with a single subapical rhinarium on each of segments 4, 6, 8 and 9; segment 10 with a bluntly rounded seta and a short bluntly rounded seta. Fore wing ( Figs 156–157 View FIGURES 150 – 157 ) 4.07– 4.43 times as long as head width, 2.64–2.87 times as long as wide, short, broad with rounded apex; vein Rs straight, slightly upturned distally, terminating short of wing apex, little shorter than vein M, RsM: 0.87–0.96; medial cell short, a little shorter than cubital cell; vein Rs short, straight, slightly upturned distally, terminating well short of wing apex; vein M longer than Rs; medial and cubital cells subequal; veins M1+2 and M3+4 short, broadly diverging with corresponding low m1 cell value: 1.47–1.50; veins Cu1a short, arched and Cu1b short, each widely divergent with corresponding low cu1 cell value: 0.97–1.16; metatibia 0.75–0.88 times as long as width of head, similar length to metafemur, without sclerotised apical spurs. Male terminalia ( Figs 176–177 View FIGURES 174 – 181 ); proctiger distinctly triangular, narrow basally, with expanded, very elongate lateral lobes bearing a prominent row of long equidistant setae on dorsoposterior margin; subgenital plate broadly rounded; parameres ( Fig. 177 View FIGURES 174 – 181 ) very long, narrow, acicular, evenly tapering to incurved sclerotised apices; distal portion of aedeagus long, with asymmetrical apical expansion ( Fig. 176 View FIGURES 174 – 181 ). Female terminalia ( Figs 140 View FIGURES 137 – 141 , 183 View FIGURES 182 – 185 ): proctiger long, elongate triangular, posterior margin with a prominent, strongly produced subterminal lobe from lateral aspect and with sclerotised apex; subgenital plate long, elongate triangular with tapering sclerotised apex; subterminal lobe of proctoger with sparse long pale setae; subgenital plate with sparse short setae.
Comments. Myotrioza oppositifoliae sp. nov. can be distinguished by the following unique combination of characters: habitus as in Figs 150–153 View FIGURES 150 – 157 , antenna with normal arrangement of rhinaria, fore wing broad with rounded apex, Rs little shorter than vein M ( Figs 156–157 View FIGURES 150 – 157 ), female proctiger with sparse setae, dorsoposterior margin with prominent subapical lobe and terminal upward inflection, valvula ventralis elongate, curved, subgenital plate acute, ventral profile of female subgenital plate curved ( Fig. 140 View FIGURES 137 – 141 , 183 View FIGURES 182 – 185 ), male proctiger triangular with long setae along dorsoposterior margin, aedeagus elongate, paramere elongate with prominent anterior subapical lobe ( Figs 176– 177 View FIGURES 174 – 181 ). For diagnosis from closely related species, see Comments for M. interstantis sp. nov.
Two males have been cited under ‘Other specimens examined’ as they could not be associated with certainty to the type material.
Etymology. Named after Eremophila oppositifolia , the host species.
Host-plant association and distribution. ( Tables 2–3). Myotrioza oppositifoliae sp. nov. is recorded from Eremophila oppositifolia R.Br. (Weeooka) from Credo Station, near Coolgardie, Western Australia. It is one of 11 species of Myotrioza gen. nov. and 17 species of Triozidae recorded for Western Australia. It is considered endemic to that state, although it is likely to occupy a broad distribution given that its hosts are widely distributed in southern Australia. It is one of 4 species, namely M. darwinensis sp. nov., M. eremophili sp. nov., M. oppositifoliae sp. nov. and M. scopariae sp. nov. from E. oppositifolia . For additional notes and distribution of its host refer to Host-plant association under M. eremophili sp. nov.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.