Membranipora de Blainville, 1830
|
publication ID |
https://doi.org/ 10.1080/00222930500415195 |
|
persistent identifier |
https://treatment.plazi.org/id/03CE7B54-FFD9-FFD2-DE8F-1F9089E1BACB |
|
treatment provided by |
Felipe |
|
scientific name |
Membranipora de Blainville, 1830 |
| status |
|
Genus Membranipora de Blainville, 1830 View in CoL
Membranipora villosa Hincks, 1880
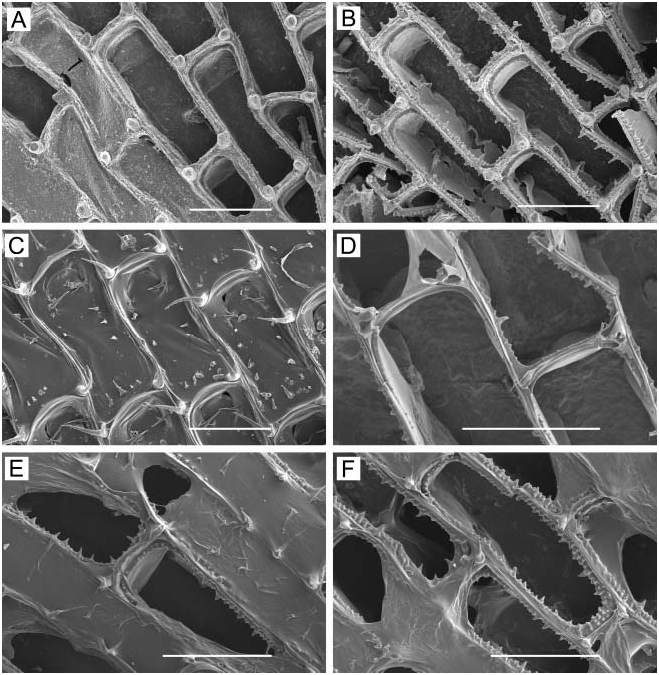
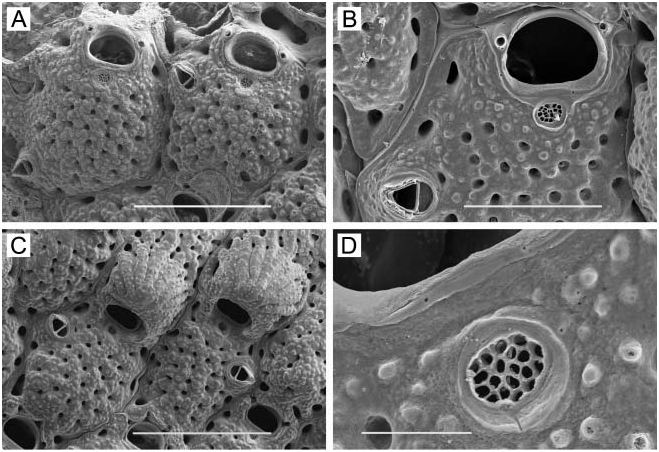
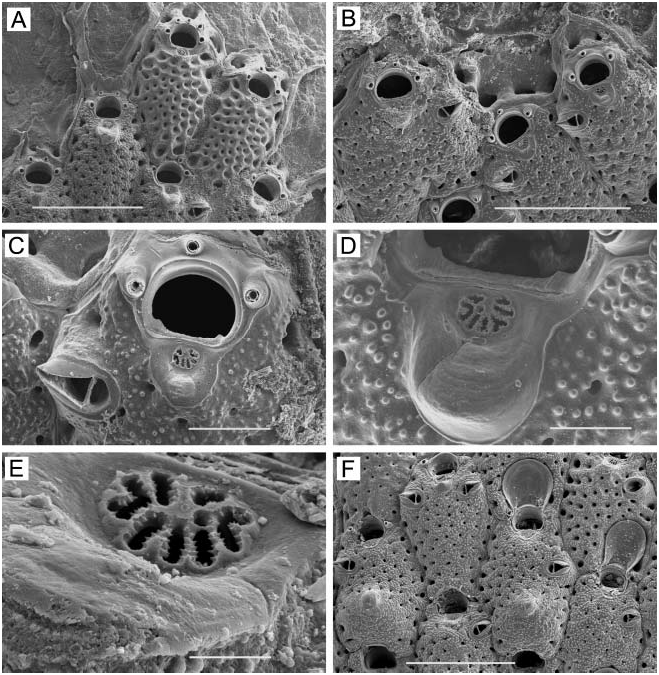
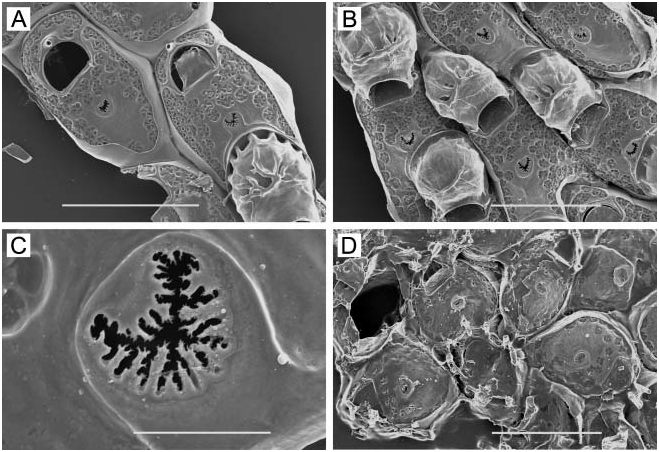
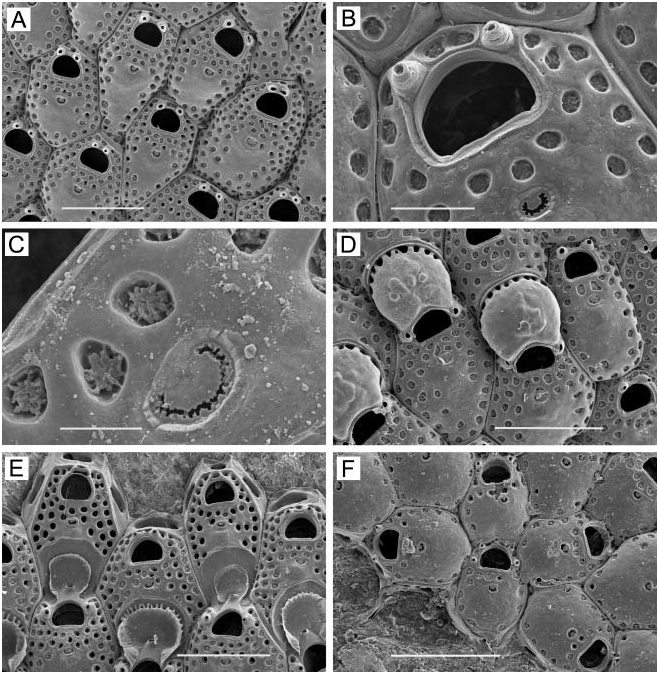
( Figure 1A–F View Figure 1 )
Membranipora villosa Hincks 1880a, p 84 View in CoL , Plate 10, Figure 8 View Figure 8 .
Membranipora villosa: Robertson 1908, p 269 View in CoL , Plate 16, Figures 22 View Figure 22 , 23 View Figure 23 , Plate 17, Figures 24 View Figure 24 , 25; O’Donoghue and O’Donoghue 1923, p 26; 1926, p 29; Soule et al. 1995, p 39, Plate 4D.
Membranipora membranacea: Hincks 1882, p 469 View in CoL ; Robertson 1908, p 267, Plate 16, Figures 19 View Figure 19 , 20 View Figure 20 ; O’Donoghue and O’Donoghue 1923, p 26; Osburn 1950, p 21, Plate 1, Figures 8 View Figure 8 , 9 View Figure 9 .
Membranipora membranacea View in CoL form serrata Hincks 1882, p 469 .
Membranipora serrata: Robertson 1908, p 268 , Plate 16, Figures 20 View Figure 20 , 21; O’Donoghue and
O’Donoghue 1923, p 26; 1926, p 29.
Membranipora serrilamella Osburn 1950, p 22 , Plate 1, Figures 12, 13 View Figure 13 .
Membranipora serrilamella: Soule et al. 1995, p 39 , Plate 4A, B.
Description
Colony. Unilaminar, encrusting, sheet-like, ranging in size from small, circular, young colonies to large expanses of coalesced colonies on laminarian algae, including plants directly accessible low in the intertidal.
Zooids. Elongate-rectangular, 0.53–1.20 mm long (average 50.849 mm, n 548, 4) by 0.23– 0.41 mm wide (average 50.290 mm, n 548, 4); closely appressed, with a narrow rim between them; walls thin and flexible or more heavily calcified and brittle; basal wall uncalcified. Frontal surface completely covered by membrane; with or without acute, short or long, flexible cuticular spines ( Figure 1C, E View Figure 1 ) arising from frontal membrane and along zooidal margins. Cryptocyst lies close below marginal rim, sometimes farther depressed distally; widest around proximal third of zooid and distal end, narrowest in central third; variably developed, consisting of a slight shelf that is smooth or weakly serrate (‘‘ membranacea ’’ form; Figure 1A View Figure 1 ), a weakly developed shelf of minute denticles replaced by longer denticles every so often (‘‘ villosa ’’ form; Figure 1D View Figure 1 ), or a pronounced shelf with smaller and longer denticles, sometimes with a pronounced denticle in proximal midline (‘‘ serrilamella ’’ form; Figure 1B View Figure 1 ). Distal cryptocyst is generally smoother than that more proximally. Distal margin sometimes extended as a raised rim.
Opesia. Differing from shape of zooidal margin by width of narrow cryptocyst; with rightangled corners proximally, or corners rounded at both ends ( Figure 1B View Figure 1 shows both cases).
Spines. Zooids have a short, blunt, hollow, conical, spine-like knob at each proximal corner ( Figure 1A, B, D, F View Figure 1 ), formed by extension and folding of the marginal wall ( Figure 1D View Figure 1 ), with closure of the fold to give a hollow process; sometimes extended with a long, curved, acute, chitinous projection (‘‘ villosa ’’ form; Figure 1C View Figure 1 ).
Avicularia. Lacking.
Ancestrula . Not observed.
Remarks
Three morphologically similar species of Membranipora have been reported in the northeastern Pacific: M. membranacea (L.) characterized by a negligible, smooth or finely serrate cryptocyst and lacking frontal cuticular spines; M. villosa Hincks characterized by a finely serrate cryptocyst and having numerous cuticular spines on the frontal membrane and zooidal margins; and M. serrilamella Osburn , characterized by a markedly serrate, spinous cryptocyst and lacking frontal cuticular spines. Yoshioka (1982) argued that these putative species represent a single species, and that the apparent difference among them are due both to ecophenotypic variation, induced partly by the presence of nudibranch predators, and to astogenetic variation. He concluded that M. villosa and M. serrilamella are synonyms of M. membranacea . However, Soule et al. (1995) retained the name M. serrilamella , arguing that it is morphologically distinct from Atlantic M. membranacea ; furthermore, they did not include M. villosa in their synonymy of M. serrilamella and provided separate illustrations of the two.
More recently, a study by Schwaninger (1999) supported Yoshioka’s results with a phylogeny of M. membranacea- and M. villosa -like morphs from Friday Harbour, Washington and M. serrilamella from Japan. Based on 600 base pairs of the mitochondrial COI gene, her phylogeny showed that single mitochondrial lineages include representatives of all three morphs. Furthermore, the three morphs proved to be genetically indistinguishable at the allozyme level, with no fixed allelic differences among them and low H values, indicating free gene flow. Schwaninger thus concluded that the three morphs are conspecific. Unfortunately, she did not show SEM illustrations of the specific morphologies included in her phylogeny. Her definition of non-‘‘ serrilamella ’’ morphs depended on the presence or absence of frontal cuticular spines, without reference to the nature of the cryptocyst, which has also been a key character in distinguishing among the nominal species reported from the north-eastern Pacific.
There is general agreement among these recent studies that M. membranacea (L.) does not occur in the north-eastern Pacific. Yoshioka (1982) noted that the cyphonautes of European M. membranacea is larger and differently ornamented than the cyphonautes attributed to the ‘‘ membranacea ’’–‘‘ serrilamella ’’–‘‘ villosa ’’ morphs in the northeastern Pacific. Schwaninger (1999) found an average COI divergence of 13% between North Atlantic and North Pacific populations of nominal M. membranacea , and concluded that these regions ‘‘harbor highly differentiated populations of M. membranacea -like morphs that may no longer belong to the same species’’. Schwaninger also noted, ‘‘there were no obvious differences in adult morphology between these populations’’, and continued to refer to Pacific populations as M. membranacea . However, Soule et al. (1995) observed differences in zooid morphology between M. membranacea they examined in France and M. serrilamella in the eastern Pacific, and concluded that these are distinct species.
At Ketchikan, we found colonies identifiable with ‘‘ membranacea ’’-like ( Figure 1A View Figure 1 ) and ‘‘ serrilamella ’’-like ( Figure 1B View Figure 1 ) morphologies, both lacking frontal cuticular spines. These are similar to one another in all respects except degree of development of the cryptocyst; the former has a negligible, faintly serrated cryptocyst, whereas the latter has a highly denticulate cryptocyst with long denticles interspersed with more numerous shorter ones. We also found a ‘‘ villosa ’’-like morph, with thinner, less heavily calcified walls having a weakly denticulate cryptocyst ( Figure 1D View Figure 1 ), and frontal cuticular spines ( Figure 1C View Figure 1 ). Another ‘‘ villosa ’’-like specimen we examined from Sitka, Alaska has abundant long, frontal cuticular spines ( Figure 1E View Figure 1 ) and more heavily calcified walls than the Ketchikan specimen, yet also shows a ‘‘ serrilamella ’’-like cryptocyst ( Figure 1F View Figure 1 ). The two ‘‘ villosa ’’-like morphs have in common a raised distal rim not present in the other morphs, but this may be an ecophenotypic character, either inducible by nudibranch predators in the same manner as the cuticular spines, or correlated with subtidal habitat. The two ‘‘ villosa ’’-like specimens encrusted laminarian algae continually submerged in a boat harbour, whereas the other morphs encrusted nearshore laminarian algae subject to wave action.
We conclude, from examining admittedly a limited number of specimens from southeastern Alaska, that there is no suite of characters that can consistently distinguish among the morphs previously reported as separate species in the north-eastern Pacific. Other authors have mentioned similar observations. For example, O’Donoghue (1926) noted, in reference to M. villosa , M. serrilamella , and M. membranacea , ‘‘A colony of one … may contain an area which, if found by itself, might easily be referred to the other’’. Similarly, Yoshioka (1982) noted, ‘‘…characteristics of all three species can be found in different parts of a single colony’’. It is also noteworthy that Osburn (1950) reported the ranges of the three nominal species in the eastern Pacific as virtually identical, from British Columbia to Southern California, with the exception of Robertson’s (1900) records of nominal M. membranacea from Yakutat and the Pribilof Islands, Alaska. Here, we consider the ‘‘ membranacea ’’, ‘‘ villosa ’’, and ‘‘ serrilamella ’’ morphs in the north-eastern Pacific as synonymous, yet distinct from European M. membranacea (L.). Nomenclatural priority therefore goes to M. villosa Hincks, 1880 , the earlier description in the eastern Pacific of either of the two morphs ( M. serrilamella and M. villosa ) other than M. membranacea .
We note, however, that questions remain concerning the identity of Membranipora spp. in the north-eastern Pacific. For instance, Osburn (1950, p 21, Plate 1, Figure 9 View Figure 9 ) noted the presence and included an illustration of ‘‘tower cells’’ for nominal M. membranacea from the Pacific. These tower zooids are characteristic of European M. membranacea (L.) ( Hayward and Ryland 1998), and their presence suggests the occurrence of that species in the Pacific, perhaps as an introduced population. Also troublesome are the deep (up to 17%) divergences in three COI lineages within a Membranipora population at a single locality, Friday Harbour, Washington ( Schwaninger 1999); these divergences are greater than that (13%) between Atlantic and Pacific populations. It is also interesting to note that although M. serrilamella has been reported from Japan ( Mawatari 1974), M. villosa has not. If the two were simply morphs of the same species, one would expect to find both in Japan. However, lack of the villosa morph there might be explained by absence ( Okutani 2000) of the specific nudibranch predators, Doridella steinbergae Lance and Corambe pacifica MacFarland and O’Donoghue ( Yoshioka 1982) , that induce chitinous frontal spines in eastern Pacific populations. This would make sense if M. serrilamella had been introduced to Japan from the eastern Pacific, in which case Japanese populations of nominal M. serrilamella might not exhibit a defensive response to chemical cues from species of nudibranch predators native to Japan. An introduction would also explain the low genetic divergence between Japanese specimens and one of the clades at Friday Harbour, Washington ( Schwaninger 1999). Ultimately, integrated morphological, ecological, and molecular genetic studies will be necessary to resolve the taxonomy of Membranipora in the northern Pacific.
Distribution
Pribilof Islands and Yakutat, Alaska (as M. membranacea ) ( Robertson 1900) southward to southern California ( Robertson 1908; O’Donoghue and O’Donoghue 1923, 1926; Osburn 1950); southern Hokkaido (as M. serrilamella ) ( Mawatari and Mawatari 1981) southward to Wakayama Prefecture on the Pacific side and Ishikawa Prefecture on the Sea of Japan, Honshu, Japan (as M. serrilamella ) ( Mawatari 1974).
Suborder NEOCHEILOSTOMINA d’Hondt, 1985
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Membranipora de Blainville, 1830
| Dick, Matthew H., Grischenko, Andrei V. & Mawatari, Shunsuke F. 2005 |
Membranipora serrilamella:
| Soule DF & Soule JD & Chaney HW 1995: 39 |
Membranipora serrilamella
| Osburn RC 1950: 22 |
Membranipora villosa:
| Soule DF & Soule JD & Chaney HW 1995: 39 |
| O'Donoghue CH & O'Donoghue E 1923: 26 |
| Robertson A 1908: 269 |
Membranipora serrata:
| Robertson A 1908: 268 |
Membranipora membranacea: Hincks 1882 , p 469
| Osburn RC 1950: 21 |
| O'Donoghue CH & O'Donoghue E 1923: 26 |
| Robertson A 1908: 267 |
| Hincks T 1882: 469 |
Membranipora membranacea
| Hincks T 1882: 469 |
Membranipora villosa
| Hincks T 1880: 84 |