Laemosaccus gossypii Hespenheide, 2019
|
publication ID |
https://doi.org/ 10.1649/0010-065X-73.4.905 |
|
DOI |
https://doi.org/10.5281/zenodo.5213750 |
|
persistent identifier |
https://treatment.plazi.org/id/DB5AFC3E-C739-5737-C0D0-E306FE12FC20 |
|
treatment provided by |
Carolina |
|
scientific name |
Laemosaccus gossypii Hespenheide |
| status |
sp. nov. |
Laemosaccus gossypii Hespenheide , new species
Zoobank.org/ urn:lsid:zoobank.org:act:A4607543-8171-4B55-9BF2-4D4C6048F38E
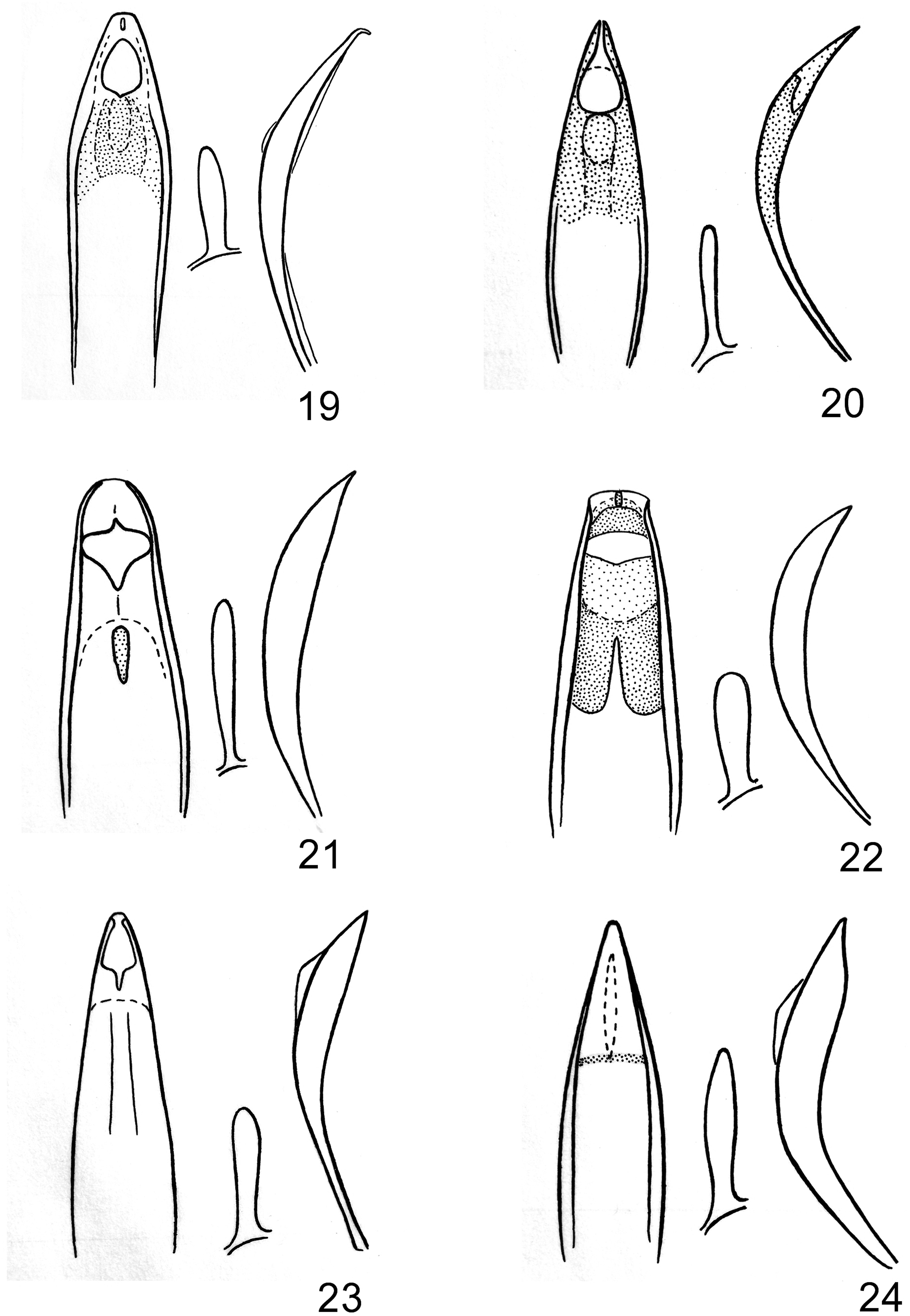
Description. Holotype Male. Length 3.1 mm, width 1.4 mm. Very similar to L. texanus ; robust, subcylindrical in cross section, broadly rounded behind, more abruptly and narrowly so in front, black throughout; head, pronotum, and elytra glabrous; metasternum and abdomen ventrally with punctures each with a small, inconspicuous silvery seta, setae more slender on femora and basal margin of tergite 7. Head hemispherical, 0.75 mm wide, rostrum rounded-terete, weakly longitudinally rugose, forming weak, irregular medial carina between even weaker lateral carinae, lateral carinae joining in weak, broad U at rostral apex, 0.55 mm long, antennae inserted slightly beyond middle. Pronotum gibbous, more or less oval, convex at base, broadest near middle, convergent toward apex, strongly, abruptly constricted before anterior margin, with lateral margins arcuate, 1.1 mm long, 1.25 mm wide, broadest just before middle, weakly convex in lateral view, very coarsely, evenly punctate, punctures rounded and separate on basal half, becoming longitudinally confluent-rugose on anterior half, rugae directed medially, with very distinct, fine, medial carina for entire length. Elytra broader than pronotum at base, narrowing then broadening again behind humeri, 1.9 mm long, 1.4 mm maximum width, elytral striae narrower than intervals, striae coarsely punctate, intervals distinctly carinate, interval 5 weakly toothed.First abdominal ventrite very slightly emarginate at midline, at posterior margin with setae less conspicuous. Profemora with broad, truncate, backward-projecting ventral tooth beyond middle and strong dorsal carina extending nearly to apex; mesofemora with small, acute, forward-projecting ventral tooth before middle, metafemora unarmed. Aedeagus 0.60 mm long, similar to that of L. texanus ( Fig. 30 View Figs ), but not conspicuously broadening at apex, apex truncate-triangular similar to that of L. andersoni ( Fig. 19 View Figs ).
Allotype Female. As male but rostrum proportionately somewhat longer, more slender, and somewhat polished, 4.1 mm long, 2.0 mm wide.
Specimens Examined. Holotype: Arizona: Superstition Mts, 4700 ft. alt., 30.12.13, iss. Feb ’14, H.S. Barber, Larvae killing Thurberia (1, USNM). Allotype: Arizona: [Maricopa Co.], Fish Creek, 2300 ft. alt., 16.12.1913, “iss. Mar-Jun,” Schwarz & Barber, Larvae killing Thurberia (1, USNM). Paratypes: USA: Arizona: S Rita Mts , Stone Cabin Ca ~ non, 26.08.1913, W. D. Pierce, on Thurberia (1, USNM), same data but no mention of host (1, USNM); Pima Co., Kitt Peak Rd. , 8.08.1976, A. E. Lewis (1, ASUHIC), Redington Pass , 16.06.1972, K. Stephan (1, ASUHIC), Santa Rita Rng Res , 21.07.1978, C. W. O’ Brien (1, ASUHIC), St. Catalina Mts. , Molino Cyn. , 8, 30.08.1969 (2, ASUHIC), Sta. Catalina Mts. , Molino Basin , 16.09.1964, C. W. O’ Brien , ex Gossypium thurberi (2, ASUHIC), Box Canyon , Santa Rita , Mts., 22.08.1965, H. R. Burke & J. R. Meyer, Taken on Gossypium thurberi Tod. (1, TAMU), Santa Rita Mts. , Box Cyn. 14.09.1964, C. W. O’ Brien , ex Gossypium thurberi (5, ASUHIC), Santa Rita R.R. , 8.08.1957, C. W. O’ Brien , Wild cotton (6, CASC; 9, ASUHIC), Baboquivari Mts , Alt. 4500, 28.04.1925, A. A. Nichol (1, USNM), Mt. Lemmon , Molino Basin , 4000’, 10.08.1964, H. R. Burke & J. Apperson (1, TAMU); Sabino Canyon , Santa Catalina Mts , 28.11.1913, iss. 02.1914, H. S. Barber, Larvae killing Thurberia (1, USNM), same data, but iss. Mar-Jun , (1, USNM); St. Catalina Mts , Molino Cyn. , 30 08.1969 (1, FSCA), 14 09.1968 (3, FSCA); Box Cyn. Rd. , 5.9 mi. NE Jcn. Whitehouse Cyn. Rd. , 31°47’57”N, 110°47’51”W, 24.09.2010, C. W. O’ Brien , beaten wild cotton, Gossipium thurberi (13, ASUHIC), 25.09.2010, 27.09.2010, C. W. O’ Brien , hand-picked ex leaf wild cotton, Gossipium thurberi (16, ASUHIC), 10.10.2010, C. W. O’ Brien , hand-picked ex leaf wild cotton, Gossipium thurberi , evening (95, ASUHIC, BMNH, CMNC, TAMU, USNM), Santa Rita Mts. , 14.09.1925, C. T. Vorhies (1, BYU). Mexico: Sonora: 9 mi. S Cananea , 16.09.1970, K. Stephan (1, FSCA); ( Son ): km 2357-8 on Interamer. Hwy. , 31.07.1962, J. M. Ramirez, On Gossypium thurberi (1, TAMU; 6, MEM), Mpio. Magdalena , N of Bella Vista (km 2344), 19.07.1962, J. M. Ramirez, On Gossypium thurberi (1, MEM), Mpio. Imuris , Intera. Hw. km 2344-2365, 6.08.1962, J. M. Ramirez, On Gossypium thurberi (9, MEM), Oquitoa , 17.08.1962, J. M. Ramirez, Cultivated cotton (1, MEM). GoogleMaps
Hosts. The genus Thurberia A. Gray , which is given on the holotype and several other collection labels, is a synonym of Gossypium . A single specimen was also taken on cultivated cotton (probably Gossypium hirsutum L.) in Sonora, Mexico.
Etymology. The species is named for the genus of its host plant, Gossypium thurberi Tod.
Discussion. Laemosaccus gossypii is very similar to L. texanus but differs in rostral and pronotal sculpture and male genitalia, uses different although closely related hosts, and apparently is separated biogeographically. Charlie O’ Brien collected them in large numbers and found that individuals became active at dusk, climbing up on the plants from beneath the plants. He wrote: “The weevils appear to be crepuscular, becoming more and more numerous as the sun goes down. I collected 75% of the specimens in the last half-hour of a 2 1/2 to 3 hour collecting stop. …Of the 120 specimens collected only one was on the underside of a leaf all others were on the tops of the leaves feeding and holding on very firmly not wishing to fall or drop from the leaves. When they do drop they fly almost immediately and try to attach themselves to grab onto another leaf lower down. …They are very strong fliers. …The leaves on the cotton are densely peppered with their feeding on the upper epidermis and the leaves are covered with fine small scars as they don’ t feed through the lower epidermis.” (C. W. O’ Brien, personal communication). There are no published reports of this species having an economic impact on cultivated cotton, although it has been collected on commercial cotton plants in Sonora, Mexico.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |