Hyalella cuyana, Alejandra & Verónica & Miranda, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5264.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:D23E1186-4A1E-48A5-8291-C9BA4B13C3D9 |
|
DOI |
https://doi.org/10.5281/zenodo.7838761 |
|
persistent identifier |
https://treatment.plazi.org/id/E047E921-FFED-FF86-FF41-7C53FDDEC6C0 |
|
treatment provided by |
Plazi |
|
scientific name |
Hyalella cuyana |
| status |
|
Hyalella cuyana View in CoL n. sp
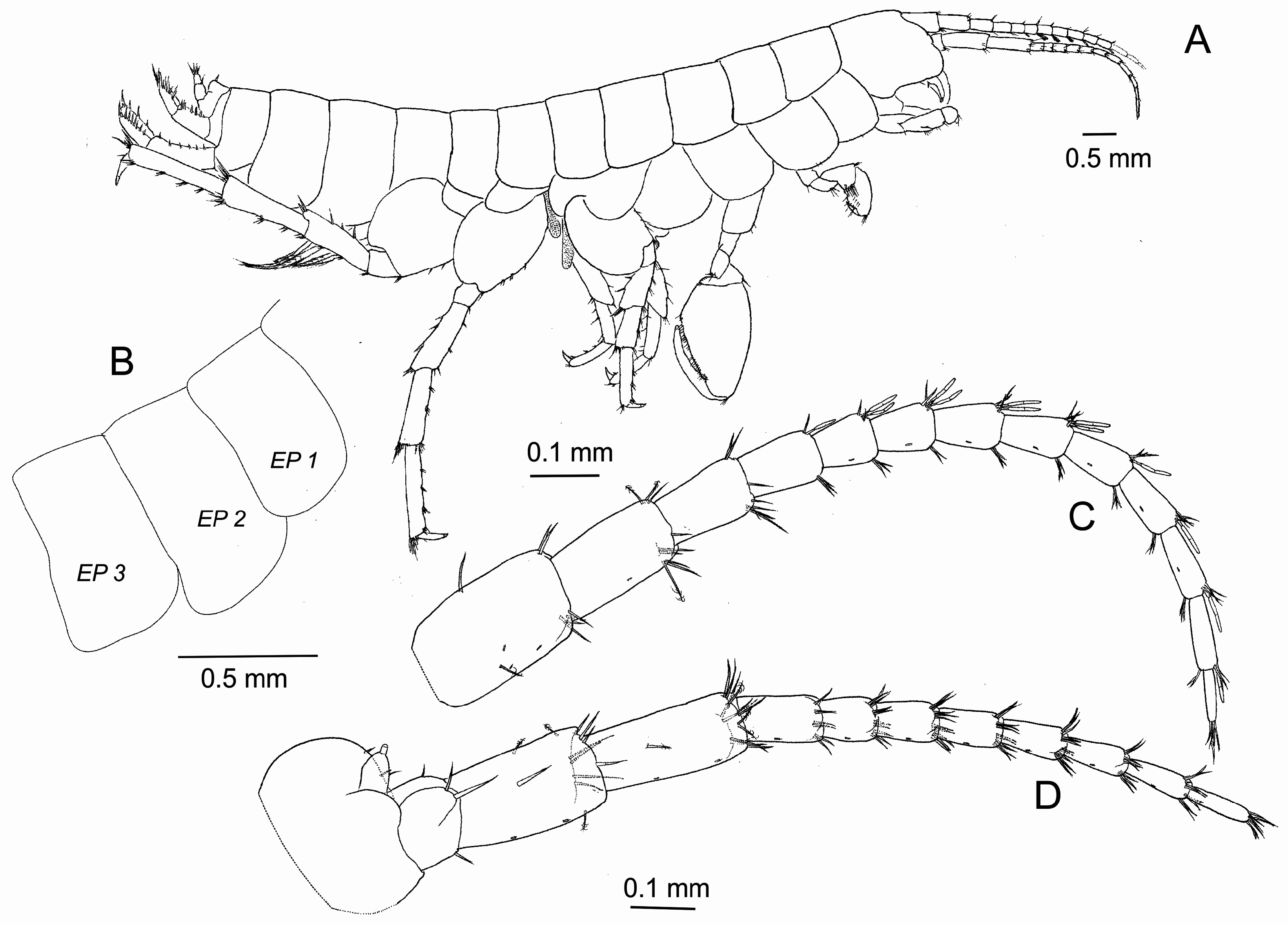
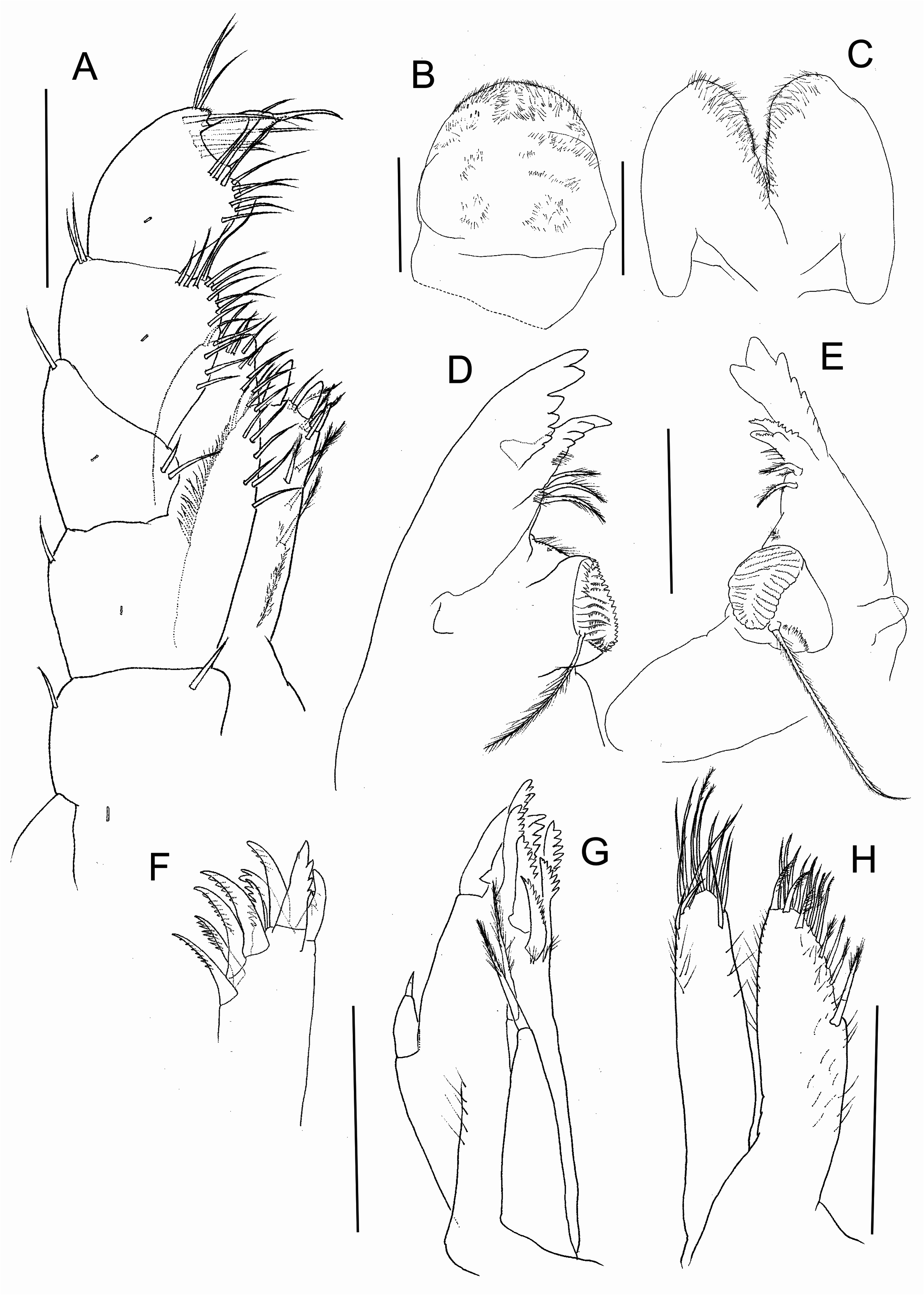
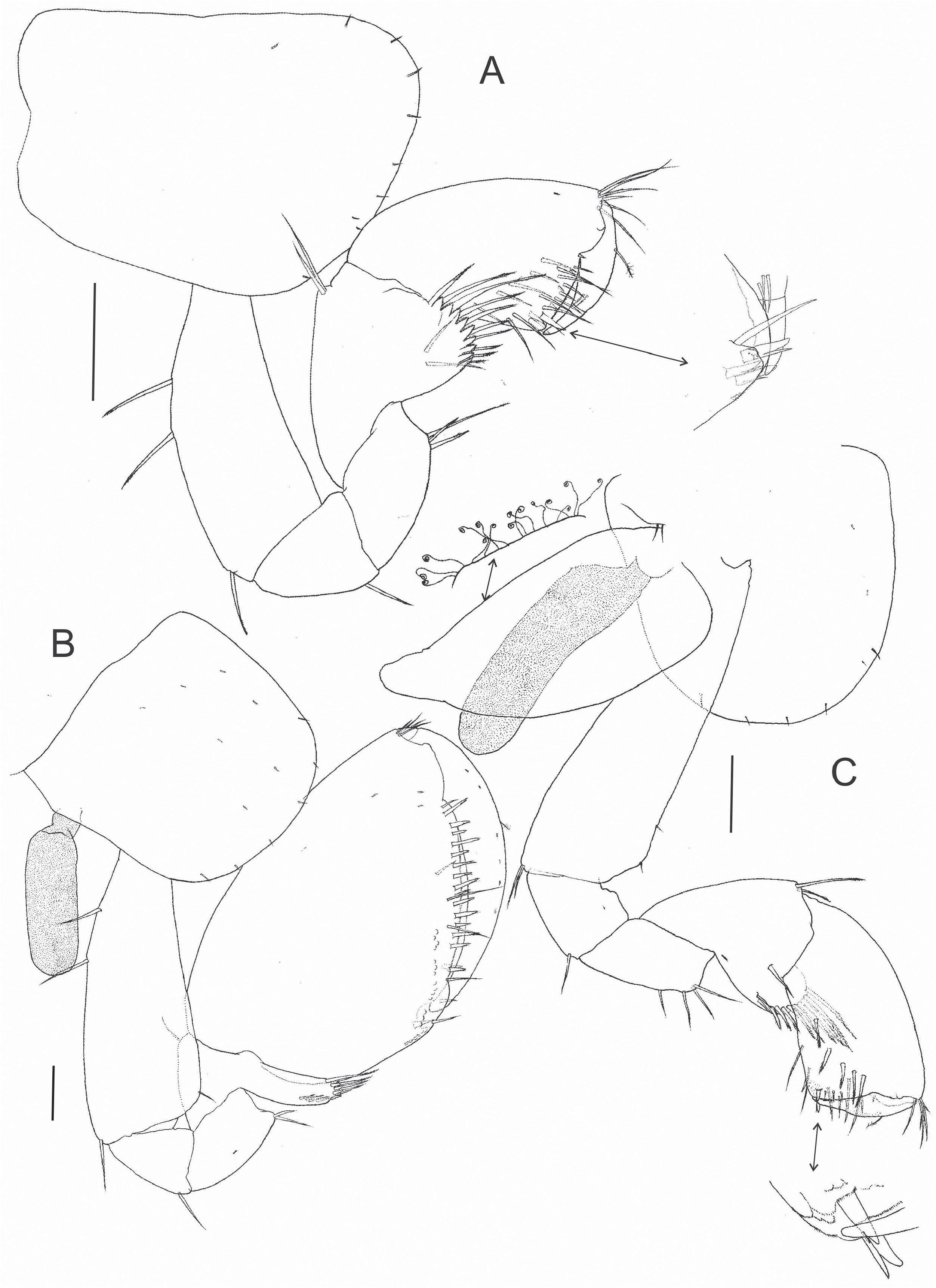
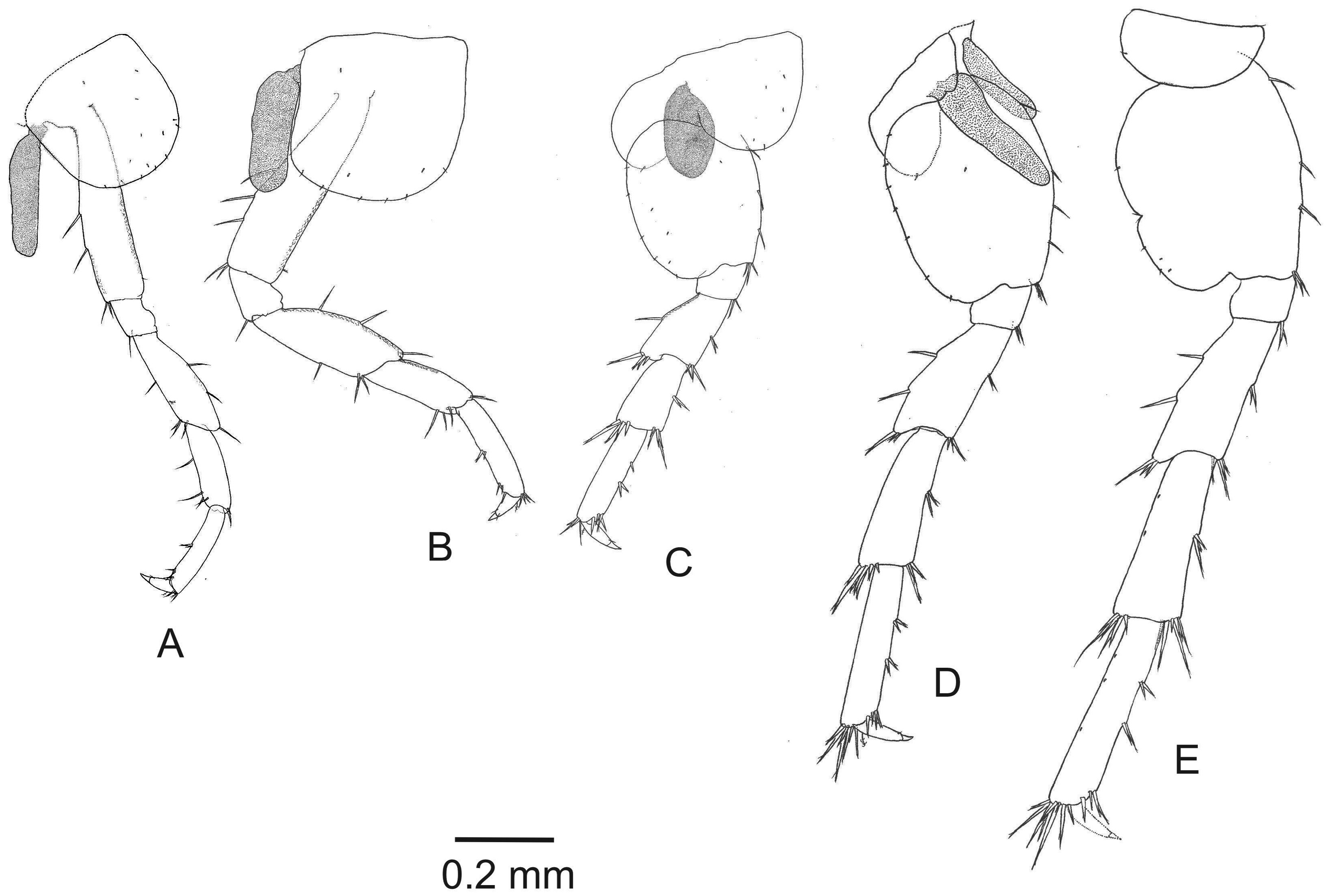
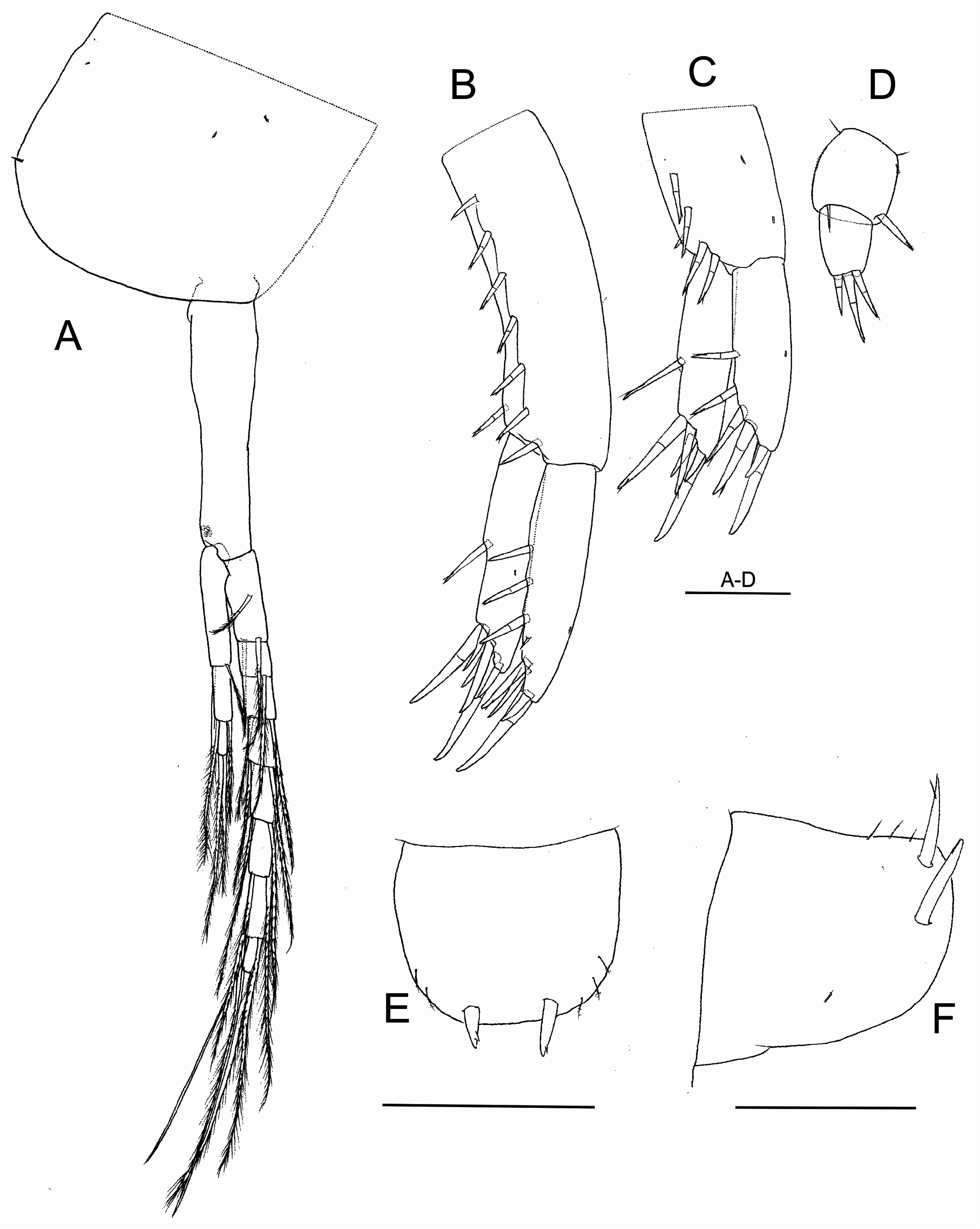
( Figs 2–8 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 )
Type Locality. Argentina, Calingasta River, San Juan Province, Calingasta Department: Calingasta River (hyporheic) Well Nº1: 31°20′30.7′′S, 69°25′38.1′′ W, 1373 m a.s.l. GoogleMaps
Material examined.
Type material. Holotype: male = 3.9 mm, FML-CRUST 01282. Paratype: 1 male = 4.3 mm, FML-CRUST 01283; 1 female ovigerous with 3 immatures in the pouch = 5.2 mm, 1 female = 4.3 mm, FML-CRUST 01284. All type materials were collected on 6 Nov 2014 by M. Peralta and A. Franck Colombres .
Additional material: 12 immatures, FML-CRUST 01285; 13 immatures, FML-CRUST 01281; 1 female = 5.08 mm and 5 immature, FML-CRUST 01291. Same collection data as the type material .
Etymology. The specific epithet is an adjective derived from the Huarpe term “cuyo” which means “land of the sands” and refers to the name of the Argentine region of Cuyo (area comprising the provinces of San Juan, Mendoza and San Luis), where the species is probably endemic.
Diagnosis. Eyes absent.Antenna 1 and antenna 2 of similar size, aesthetascs elongated. Coxal plate of pereiopod 4 posteriorly without excavation. Epimeral plates 1–3 rounded posterodistally. Male gnathopod 2 propodus almondshaped, ovate, palm slope strongly oblique. Pleopods inner ramus reduced to two articles in pleopod 1 and three in pleopods 2–3.
Description of holotype male. Body length 3.9 mm ( Fig. 2B View FIGURE 2 ). Head smaller than the first two thoracic segments combined. Eyeless. Body surface smooth. Epimeral plates 1–3 with rounded posterodistally ( Fig. 3B View FIGURE 3 ). Pereiopodal coxae 1 to 3 subequal in shape, slightly over-lapping. Coxal plates 1–4 in lateral view, as deep as the associated pereionite.Acumination in coxae absent. Coxa 3 narrower than 4. Coxa 4 subquadrate, without posterior excavation. Posterior lobe of coxa 5 deeper than anterior lobe. Anterior lobe of coxa 6 small ( Figs 6A–D View FIGURE 6 ).
Antenna 1 ( Fig. 3C View FIGURE 3 ) subequal in size to antenna 2. Peduncle slightly shorter than head, peduncular segments 1–3 progressively shorter, all segments with simple and plumose setae. Flagellum with ten articles bearing groups of simple setae, additionally with aesthetascs occurring distally between articles 1–9, elongated, reaching about ½ to 3 / 4 length of the antennal article.
Antenna 2 ( Fig. 3D View FIGURE 3 ) less than half of body length. Peduncle longer than peduncle of antenna 1, peduncular segments 4–5 of similar length with simple and plumose setae. Flagellum with eight articles bearing groups of simple setae.
Labrum ( Fig. 4B View FIGURE 4 ) anterior margin covered sparsely by simple setules.
Mandibles basic amphipodan type (sensu Watling 1993). Right mandible ( Fig. 4E View FIGURE 4 ) with incisor 8-denticulate (three long, five short), lacinia complex, setal row with two pappose setae. Left mandible ( Fig. 4D View FIGURE 4 ) with incisor 7- denticulate (three long, four short), lacinia 4-denticulate, setal row with three pappose setae. Both mandibles have well-developed, cylindrical, triturative molar; mandibular palp rudimentary, reduced to a small blunt process.
Lower lip ( Fig. 4C View FIGURE 4 ). Outer lobes and mandibular process rounded; lobes covered by sparse setules. Inner lobes wanting.
Maxilla 1 ( Figs 4F–G View FIGURE 4 ). Palp short, unsegmented, longer than wide, reaching less than half the length of the distance between the base of the palp and the apex of the outer plate, with a strong distal seta. Inner plate shorter than outer one, with two apical papposerrate setae, and several marginal setules. Outer plate with nine stout serrate setae.
Maxilla 2 ( Fig. 4H View FIGURE 4 ), both plates subequal in size. Inner plate with one strong, long papposerrate seta proximally on inner margin. Outer and inner plate sparsely setulose, with apex wearing simple, serrulate, pappose and serrate setae as figured.
Maxilliped ( Fig. 4A View FIGURE 4 ). Inner plate longer than wide, reaching ¾ of length of outer plate, with three long robust cuspidate setae apically and other pappose and simple setae and setules on apex and margins; outer plate with apical and medial simple setae; palp 4-segmented, second segment longer than wide, all segments with simple or serrate setae on inner and outer margins; third segment with distal and inner margins with cluster of several simple or serrate setae; fourth segment unguiform with long distal nail.
Coxal gills sacciform, present on gnathopod 2 ( Fig. 5B View FIGURE 5 ) to pereiopod 6 ( Fig. 6A–D View FIGURE 6 ). Sternal gills tubular, present on pereonites 3 to 7.
Gnathopod 1 ( Fig. 5A View FIGURE 5 ) subchelate. Coxal plate longer than broad, margin with small simple setae. Basis, ischium and merus with 1–2 serrate setae on posterior margin. Carpus longer than wide, concave posterior lobe bordered by pectinate and serrate setae. Propodus longer than its maximum width, hammer-shaped, without comb/ setose-scales, anterodistal border bearing a group of thin simple setae, inner face with five serrate setae in oblique row; palm slope slightly transverse; palm angle with three endal, cuspidate setae with accessory seta. Claw-like outer margin of dactylus with one thin plumose seta dorsally, inner margin with denticles. Palmar Index (sensu Ruffo 1973) = 0.46.
Gnathopod 2 ( Fig. 5B View FIGURE 5 ) subchelate. Coxal plate narrow, about as long as wide, with thin setae. Basis, ischium and merus with 1–2 simple setae on the posterior margin. Posterior lobe of carpus elongated, margin pectinate with some serrate setae. Propodus ovate, almond-shaped, palm slope strongly oblique, margin evenly and slightly convex, fringed by numerous apical setae, some cuspidate with accessory seta and others simple. Dactylus claw-like congruent with palm, with one thin plumose seta dorsally. Palmar Index (sensu Ruffo 1973) = 0.63
Pereiopods 3 and 4 ( Figs 6A–B View FIGURE 6 ), similar in size and shape. Coxal plates sub-quadrate in shape, coxa 4 posteriorly without prominent excavation; merus, carpus and propodus posterior margin with simple setae, additionally merus of pereiopod 3 and basis of pereiopod 4 with plumose seta. Dactylus less than half of propodus length.
Pereiopods 5–7 ( Figs 6C–E View FIGURE 6 ). Pereiopods 6–7 significantly longer than pereiopod 5. Coxal plate of pereiopod 5 bilobed with one seta on anterior and posterior lobe, basis anterior margin with two to three serrated setae; coxa of pereiopod 6 weakly bilobed with one seta on posterior lobe; pereiopod 7 coxa wider than long, basis posterior margin with a notch bearing two thin simple setae. Anterior and posterior margins of merus, carpus and propodus of pereiopods 5–7 with marginal clusters of 2–8 cuspidate setae. Basis posterior margin of pereiopod 7 with a notch bearing two thin simple setae.
Pleopods ( Fig. 7A View FIGURE 7 ). Peduncle with retinacula. Biramous, inner ramus shorter than peduncle, reduced to two articles in pleopod 1 and three in pleopods 2 to 3, outer ramus of pleopods 1–3, with 7 articles, rami bearing long plumose setae.
Uropod 1 ( Fig. 7B View FIGURE 7 ). Peduncle 1.5 times longer than rami, with a dorsal row of six cuspidate setae with accessory seta, and others two distal setae; rami subequal in size; inner ramus with one dorsal and six distal cuspidate setae (two of them much longer), modified curved seta on inner side of ramus wanting; outer ramus with 3–4 dorsal and four distal cuspidate setae, one of them longer.
Uropod 2 ( Fig. 7C View FIGURE 7 ) shorter than uropod 1. Peduncle slightly shorter than rami, with four cuspidate setae with accessory seta; inner ramus with one dorsal and five distal cuspidate setae; outer ramus with two dorsal and four distal cuspidate setae, one of them shorter.
Uropod 3 ( Fig. 7D View FIGURE 7 ) length similar to peduncle of uropod 2; peduncle as wide as long, with two distal setae, one cuspidate and other simple; ramus shorter than peduncle, with three distal cuspidate setae.
Telson ( Figs 7E–F View FIGURE 7 ) wider than long, entire, with two robust apical cuspidate setae and 2–3 very slender simple lateral setae arranged in a submarginal row.
Female ( paratypes). Body larger than male, mean body length 4.86 mm (n = 3). Habitus similar to male except for gnathopod 2, which displays more elongated propodus, inner face of propodus with 5–6 serrate setae in oblique row ( Fig. 5C View FIGURE 5 ) (right propodus with six). Oostegites subtriangular, in one female mature (body length 5.2 mm) are equipped with curled setae on the margins, in other female (body length 4.3 mm) without such setae. Antenna 1 flagellum with 11–14 articles. Antenna 2 flagellum with 11 articles. Suspected sexual differences in the number of setae on uropods 1–2 rami (females with an additional dorsal seta in relation to the males) pending confirmation given that only a small sample of adult specimens was available.
Habitat. Freshwater, hyporheic.
Taxonomic remarks. Hyalella cuyana n. sp. can be distinguished from the other species of the genus by the combination of characters given in the diagnosis, some of which differ from the diagnosis of Hyalella and the description of generic characters provided by González & Watling (2001): Antennae 1 and 2 subequal in size, and coxal plate of pereiopod 4 posteriorly without excavation. In relation to the latter characters, in epigean species H. pauperocavae González & Watling, 2002 the coxal plate 4 also presents an uncommon shape for the genus, with a slightly excavated posterior margin, and its antennae are subequal in size. Hyalella pauperocavae shows some other similarities with H. cuyana n. sp., namely, sternal gills present on pereonites 3–7; palp of maxilla 1 longer than wide, reaching less than half the distance between base of palp and tip of setae on outer plate; gnathopod 2 palm slope oblique; outer ramus of uropod 3 shorter than peduncle; and modified curved seta on inner side of ramus wanting. However, H. pauperocavae is distinguished from H. cuyana n. sp. by having pigmented eyes, the maxilliped inner plate with three not very long cuspidate setae apically, inner surface of gnathopod 1 propodus with ten serrated setae (vs five setae), telson with six to eight setae distributed asymmetrically on apical margin (vs two setae), well developed pleopods, and pereiopods 6 and 7 not significantly longer than pereiopod 5.
In its general morphology, H. cuyana n. sp. is similar to other hypogean congeneric species by presenting: reduction of ommatidia or total absence of eyes (except in H. paramoensis Andres, 1988 ); dorso-posterior carina on pleonite 1–3 absent (except H. cenotensis Marrón-Becerra, Hermoso-Salazar & Solís-Weiss, 2014 ); two terminal setae on inner plate of maxilla 1, typical of the genus Hyalella (except H. cenotensis ); gnathopod 2 of male without lobiform process on palmar corner (except H. muerta Baldinger, Shepard & Threloff, 2000 ); reduction of the size of uropod 3; few or no apical setae on telson; reduction of inner ramus of pleopod (only in H. rionegrina Grosso & Peralta, 1999 ; H. muerta and H. cuyana n. sp.); attenuation of the coxae plates, especially in pereiopod 4 (only in H. muerta and H. cuyana n. sp.), it should be noted that these features are not mentioned or illustrated in the original descriptions of H. formosa Cardoso & Araujo, 2014 in Cardoso et al. (2014), H. spelaea Bueno & Cardoso, 2011 in Cardoso et al. (2011) or H. caeca Pereira, 1989 . H. veredae Cardoso & Bueno, 2014 in Cardoso et al. (2014) is the only Hyalella stygobiont species that shares with the new species the character of antennae 1 and 2 subequal in length, although it is easily distinguishable by the posterior excavation of the coxal plate 4 (not developed in H. cuyana n. sp.) and the modified curved setae on the inner ramus of uropod 1 of the male (absent in H. cuyana n. sp.). Furthermore, the new species has elongated aesthetascs (similar to H. epikarstica Rodrigues, Bueno & Ferreira, 2014 ; H. formosa ; H. veredae and H. troglofugia Bastos-Pereira, De Oliveira & Ferreira, 2018 ). The newly described species is similar to H. epikarstica and H. troglofugia in having the maxilliped inner plate with three very long cuspidate setae apically. Furthermore, H. muerta has the male gnathopod 2 palm excavated, which makes it easily distinguishable from H. cuyana n. sp.
The distribution of sternal gills in Hyalella is generally used when describing and comparing species. In H. cuyana n. sp.; H. montana Rodrigues, Senna, Quadra & Bueno, 2017 ; H. epikarstica ; H. imbya Rodrigues & Bueno, 2012 in Rodrigues et al. (2012); and H. muerta the sternal gills are developed on pereonites 3–7. Table 1 presents a detailed morphological comparison between the 14 stygobiont or stygophile Hyalella species that are in the process of adaptation to the hypogean habitat ( Rodrigues et al. 2017). Some of the characters included in Table 1 have not been included in the species original descriptions (e.g. development of aesthetascs; shape of coxal plate 4) or are male characters that are unknown in H. rionegrina and H. anophtalma Ruffo, 1957 (for which only females are known).
The morphology of the gnathopod 2 propodus of male H. cuyana n. sp. is similar to those of H. meinerti Stebbing, 1899 (epigean); H. caeca (cavernicolous); and H. imbya (hypothelminorheic). These species share the condition of having a strongly oblique palm slope in the gnathopod 2 propodus of males. However, H. meinerti and H. imbya differ from the new species by having the coxal plate 4 excavated posteriorly. H. meinerti differ from the new species in having well developed eyes; antenna 1 shorter than antenna 2; uropod 3 outer ramus longer than peduncle, and arrangement of the telson (without lateral setae). H. imbya is differentiated from the new species mainly in having antenna 1 longer than antenna 2; very short palp of maxilla 1; inner face of gnathopod 1 propodus with seven serrated setae (vs five setae). The new species also differs from H. caeca mainly in having antenna 1 much shorter than antenna 2 (antennae 1 and 2 similar in H. cuyana n. sp.); inner face of gnathopod 1 propodus with seven serrated setae (vs five setae); telson with two short apical setae (vs two long and strong apical setae and three lateral setae).
Comments about adaptation of Hyalella cuyana n. sp. to subterranean environments. The animal species that inhabit caves and other subterranean (aquatic and terrestrial) environments exhibit similar reductive ( Romero & Green 2005) and constructive traits ( Jones & Culver 1989) arisen via convergent evolution. The repeated independent evolution of such traits in different taxa provides strong evidence that they are related to a common environmental condition, i.e. the complete absence of light, shared by subterranean taxa ( Carlini & Fong 2017). These convergences are a potential critical issue in the reconstruction of phylogenetic relationships in groups with epigean and hypogean species, as is the case of Hyalella .
The absence of eyes in Hyalella is typical of obligate groundwater-dwelling species ( Bastos-Pereira et al. 2018), but this pattern presents some exceptions such as, for example, the presence of eyes in H. cenotensis which was collected in open water (accidental occurrence?) or the reduced number of ommatidia in the eyes of H. spelaea (cavernicolous).
In almost all the species related to subterranean habitats included in Table 1, the pereiopods 6–7 are significantly longer than pereiopod 5. Elongation of appendages is a widespread trend in stygobiont amphipods ( Väinölä et al. 2008). Likewise, the increased length of the thoracic appendages and antennae of cave-dwelling species has traditionally been explained as a consequence of selection for non-visual senses and an increased ability to find food in a low-energy environment ( Culver & Pipan 2009; Culver et al. 2010; Hüppop 2000). Nevertheless, results from Delić et al. (2016) suggest that, rather than being a general adaptation to cave life, long appendages such as pereiopods 5–7, appear to be associated with the absence of water flow, as well as with character displacement when in sympatry with ecologically similar competing species.
Regarding the development of sensory structures, the antennae of H. cuyana n. sp. bear long aesthetascs. These are complex sensilla harboring chemoreceptor neurons (Tiernery et al. 1986; Sandeman & Sandeman 1996). Studies by Ziemba et al. (2003) have shown that the length of individual aesthetascs of cave-dwelling crayfish of the genus Orconectes was greater in O. australis packardi compared to the surface-dwelling species Orconectes cristavarius . These observations suggest that the neurons of O. a. packardi may be exposed to more chemical signals, which could favor the specialization for greater sensitivity. In the case of Hyalella , this raises the question, to be explored in the future, whether there is a relationship between the length of aesthetascs in hypogean species and environmental variables.
In addition, stygobiont amphipods like H. cuyana n. sp. show structural rudimentation, e.g. in the pleopods. According to Botosaneanu (2001) the reduction of pleopod size can be associated, at least in many cases, with reduction or loss of the natatory ability of these appendages.
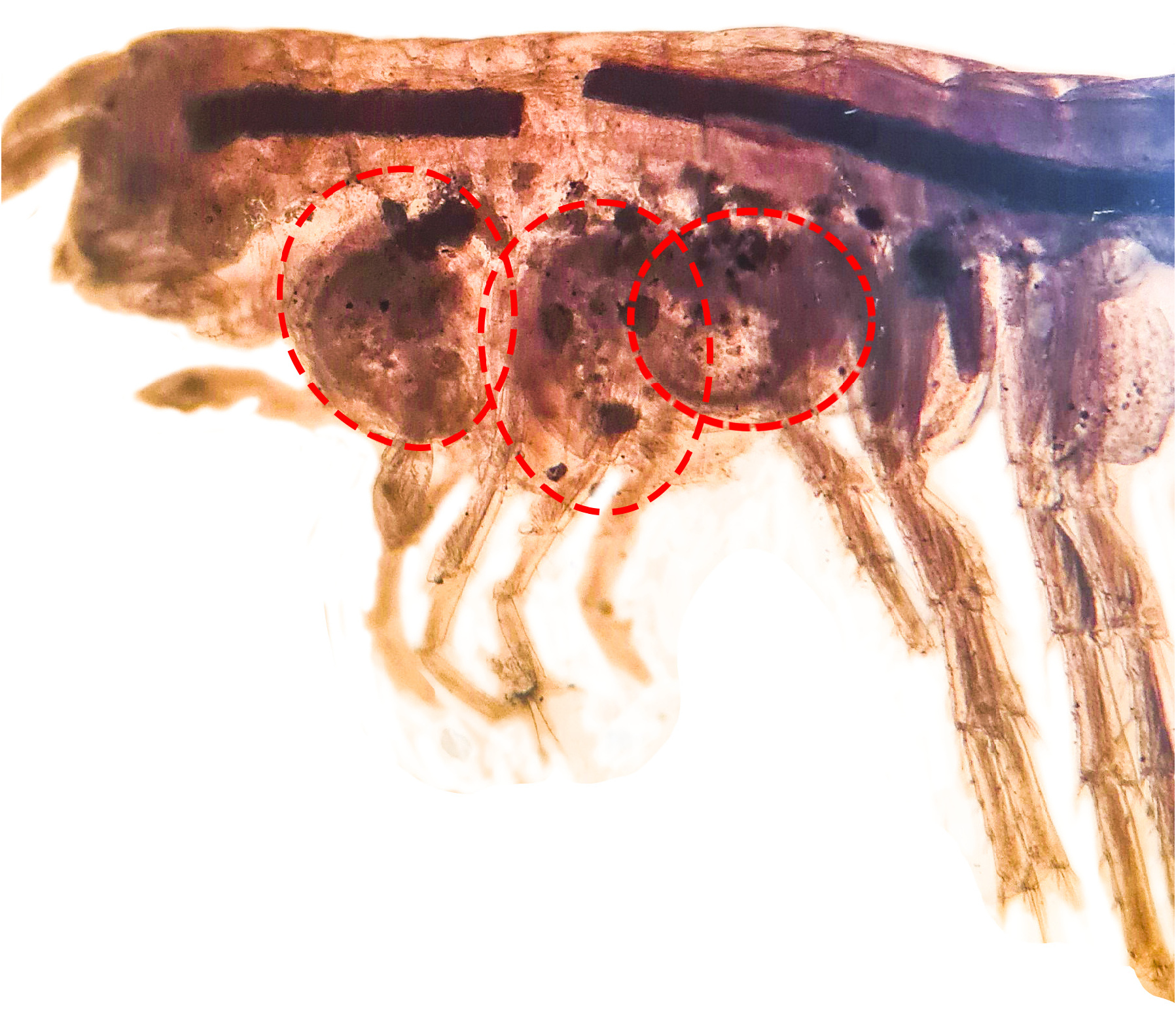
Only tree prejuvenile individuals were observed in the marsupium of the female ovigerous paratype of Hyalella cuyana n. sp. ( Fig. 8 View FIGURE 8 ). In the description of the only known ovigerous female of H. anophthalma, Ruffo (1957) comments that the low number of eggs found in the pouch, in combination with the considerable size of the eggs themselves, may be also considered characteristics connected with the subterranean habitat. Additionally, fewer and larger eggs and offspring have also been reported in other subterranean amphipods. Fišer et al. (2012) found that cave-dwelling Niphargus females lay more voluminous eggs and their brood volume was significantly larger. Larger Niphargus females appear to allocate excess energy to more voluminous eggs rather than to a greater number of eggs. The volume of an egg often correlates positively with its nutritional value, which lengthens the development within the egg. In turn, this results in larger juveniles, with greater fitness and probably greater ability to cope with the challenges of the subterranean environment.
Further studies are needed to elucidate how local and specific abiotic and biotic conditions of sub-superficial environments, such as hyporheic habitats, may be related to particular features of the Hyalella species that inhabit them.
Distributional and biogeographical remarks on Hyalella cuyana n.sp. The specialized sub-surface stygobiont Hyalella cuyana n. sp. is probably endemic to the Eremean district of the Monte province, within of the South American Transition Zone (see biogeographic provinces of Argentina in Arana et al. 2021). The Eremean district is a narrow strip along the high pre-Andean valleys, in Mendoza and San Juan provinces. According to Elías & Aagesen (2016), it is similar to the “Uspallata-Calingasta” natural area proposed by Roig Juñent et al. (2001) based on the distributional patterns of several endemic insect species. In relation to the factors that might lead to faunal diversification in groundwaters on an evolutionary scale, Gibert et al. (1994) hypothesized that the great age and the relative constancy and predictability of habitat conditions should be conducive to speciation. Throughout geological history, southern South America has undergone different geological processes and climatic events: Andean orogeny; glaciations; marine introgressions; drainage reversals, etc. that were decisive for the distribution of its biota ( Collado et al. 2021). Cannizzaro & Berg (2022) using a molecular phylogeny of the superfamily Hyaloidea , concluded that the lineage that would give rise to the freshwater hyalellids split from their marine ancestors in the late Mesozoic, during the Cretaceous (~110 Ma ± ~30 Ma).
Hyalella cuyana n. sp. occurs in sympatry with other stygobiont crustaceans ( Isopoda and Anaspidacea ) belonging to groups with different phylogenetic ages and colonization processes of limnic environments ( Holsinger 1994; Notenboom 1991). Based on their common occurrence, we could hypothesize that the intermountainous Calingasta Valley is part of the ancient groundwater basin of western South America south of 28ºS since this valley harbors interstitial fauna of great and disparate antiquity ( Peralta 2014).
Conservation. The Andean provinces of central-western Argentina, namely Mendoza and San Juan, are among the most vulnerable areas in the country with respect to global climate change, as they are highly dependent on the water from the Andes. In these provinces, the last decades have witnessed a reduction of the flow of rivers and the consequent decrease in the fluvial contribution to aquifers. This has had negative effects on the surface water supply during the summer, when there is greater demand for water as a resource for various uses (mining, agriculture, domestic usage, etc.). This situation generates greater demand for groundwater extraction, in turn negatively affecting its stygofauna.
TABLE 1. (Continued)
TABLE 1. (Continued)
TABLE 1. (Continued)
TABLE 1. (Continued)
At the local scale, and specifically in 2015 after the sampling we carried out along the Calingasta River, we recorded several transformations of the riparian landscape. Funded by both governmental and private sources, numerous hydraulic constructions ( Fig. 9 View FIGURE 9 ) were carried out with the goal of widening and profiling the channel that redirects part of the Calingasta River course, approximately from the water intake at Villa Calingasta to its mouth into the Los Patos River; including the construction of gabion mattresses on both banks of the riverbed. These hydraulic works were partly a response to the remediation for mine tailings dumps. Removal of the bed and banks of a reach of the Calingasta River were observed in the proximity of the sampling site for crustaceans described here.
The abovementioned infrastructure works that affect the River, forced us in October of 2015 to move the sampling of interstitial fauna upstream; however, no stygobiont crustaceans were found in that sampling. These landscape modifications and the consequent habitat fragmentation, combined with climatic changes (low rainfall), are key factors in the loss of groundwater species.
Thus, it becomes strongly necessary to assess the changes in hyporheic biodiversity that took place after the infrastructure works on the Calingasta River should be evaluated after the infrastructure works developed in the River. Among the hyporheic taxa that may have been most affected by these environmental changes are endemic species such as H. cuyana n. sp. For these reasons we propose that H. cuyana n. sp. should be classified as Critically Endangered (CR) according to the IUCN (2022) criteria B1a (the new species is known at only a single location); and B1b iii (continuing decline, observed, inferred in the quality of habitat) because the new species occurs in a specialized and vulnerable sub-superficial habitat. The type locality of H. cuyana n. sp. is in a periurban area exposed to several anthropogenic phenomena that result in a decline of habitat quality (including habitat fragmentation, removal of the River bed and banks, channel building, River flow regulation, pollution of superficial and sub-superficial water from land-use and mining, over-exploitation of aquifers). This classification as Critically Endargered is similar to the one assigned to the Brazilian stygobiont species H. veredae ( Zepon et al. 2021) and H. caeca ( Pereira 1989) from Alto do Ribeira karst area, S„o Paulo.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Senticaudata |
|
SuperFamily |
Hyaloidea |
|
Family |
|
|
Genus |