Gorgocephalus graboides, Huston & Cutmore & Miller & Sasal & Smit & Cribb, 2021
|
publication ID |
https://doi.org/10.1093/zoolinnean/zlab002 |
|
publication LSID |
lsid:zoobank.org:pub:AAA956A8-14F7-49E4-888F-072FAC7D3826 |
|
DOI |
https://doi.org/10.5281/zenodo.5761796 |
|
persistent identifier |
https://treatment.plazi.org/id/D684AF8B-5CF6-40A0-91AD-7B26A304D47B |
|
taxon LSID |
lsid:zoobank.org:act:D684AF8B-5CF6-40A0-91AD-7B26A304D47B |
|
treatment provided by |
Plazi |
|
scientific name |
Gorgocephalus graboides |
| status |
sp. nov. |
GORGOCEPHALUS GRABOIDES View in CoL SP. NOV.
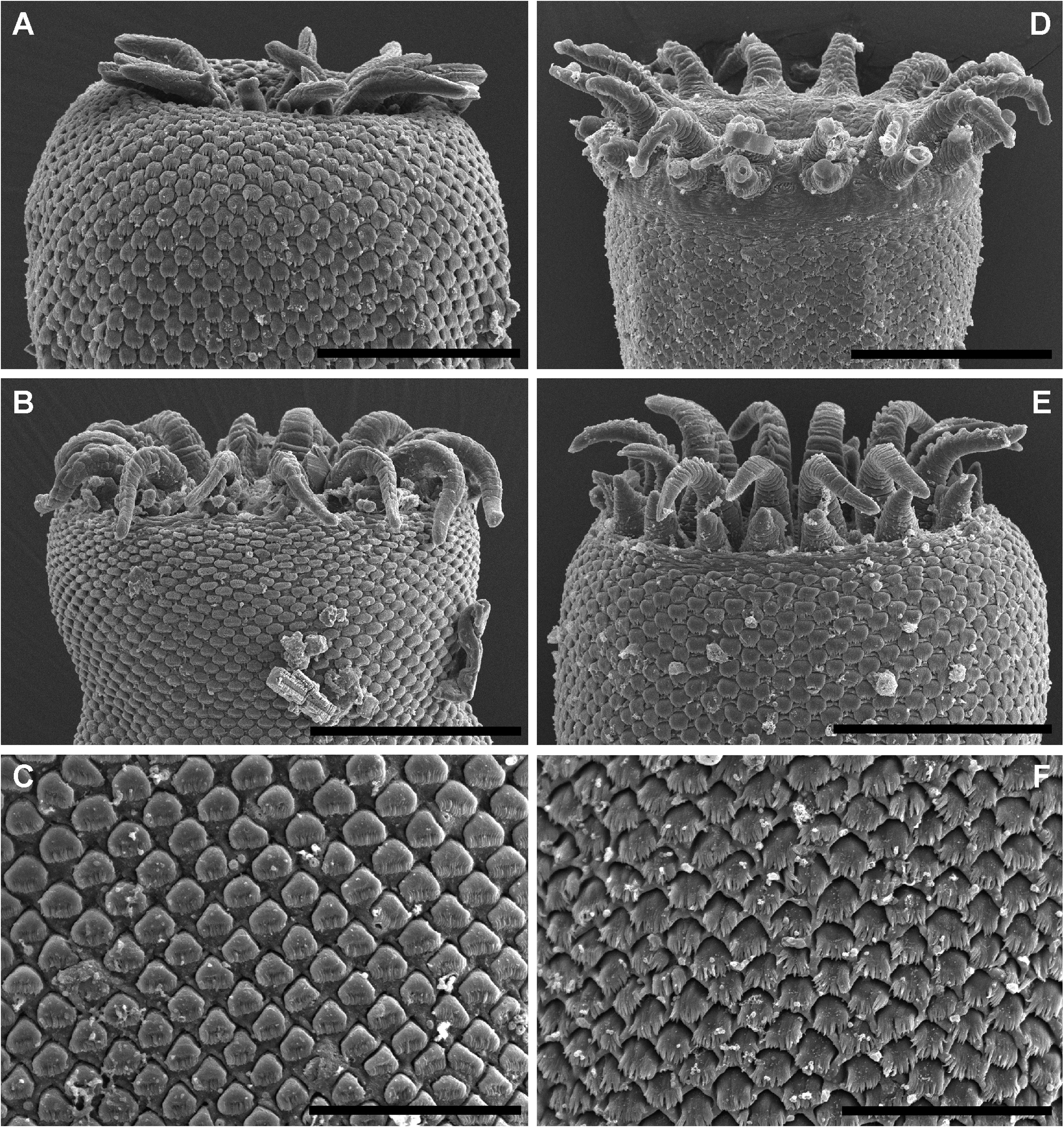
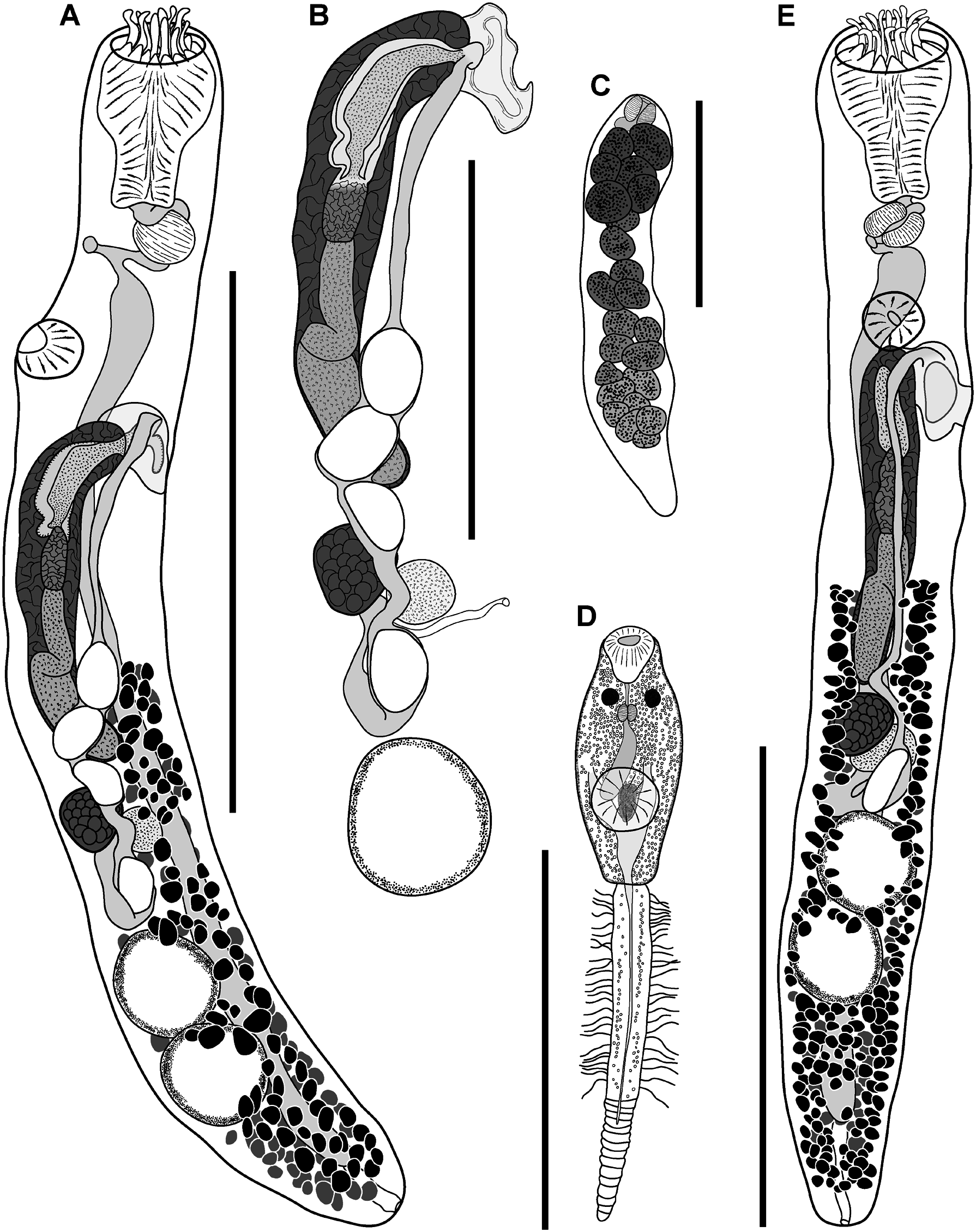
( FIGS 12D–F View Figure 12 , 13 View Figure 13 ; TABLES 4 View Table 4 , 6)
Z o o b a n k r e g i s t r a t i o n: u r n: l s i d: z o o b a n k. org:act: D684AF8B-5CF6-40A0-91AD-7B26A304D47B.
Type host and locality: Kyphosus cinerascens (Forsskål, 1775) , highfin chub ( Perciformes : Kyphosidae ) from off Lizard Island, Great Barrier Reef, Queensland, Australia ( 14°41’10’’S, 145°28’15’’E).
Other hosts (intermediate): Echinolittorina vidua (G o u l d, 1 8 5 9) (G a s t r o p o d a: L i t t o r i n i m o r p h a: Littorinidae ).
Type material: Holotype ( QM G238605 ) ex K. cinerascens from off LI . Paratypes: 14 whole mount and three hologenophore specimens ex K. cinerascens from LI ( QM G238606 – G238622 ) .
Additional voucher material (intramolluscan): Five slides of cercariae and rediae ex E. vidua from LI (QM G238623–G238627).
Site in host: Pyloric caeca (definitive); gonad/digestive gland (intermediate).
Representative DNA sequences: Six sequences deposited for COI mtDNA ( MW353677 View Materials – MW353682 View Materials ); six sequences deposited for 5.8S-ITS2-partial 28S rDNA ( MW353959 View Materials – MW353964 View Materials ); four sequences deposited for partial 28S rDNA ( MW353905 View Materials – MW353908 View Materials ); see Supporting Information, Table S2 View Table 2 .
Etymology: This species is named for its resemblance to the monsters from the Tremors films, ‘graboids’. Graboids are blind, subterranean, worm-like predators with grasping tentacles that emerge from their beaklike maws. Graboids even have complex life-cycles, transitioning through several radically different phenotypes. The name is formed by combination of ‘graboid’ and a Latinized Greek suffix indicating a resemblance or likeness, ‘oides’.
Description of adult ( Figs 12D–F View Figure 12 , 13A, B, E View Figure 13 ): Measurements in Table 4 View Table 4 . Description based on type material and SEM images of three adult specimens. Body elongate, cylindrical, broadest in region of ventral sucker, tapering slightly posteriorly. Tegument armed with alternating rows of partially overlapping comblike scales; distal portion of scales forming up to 15 distinct tendrils. Eyespot pigment sparsely scattered in forebody and anterior third of hindbody. Oral sucker terminal, partially retractable, infundibuliform, broadest in anterior region with distinct reduction in diameter about mid-length continuing through to posterior margin; margin of anterior portion bearing crown of 14 bifid tentacles; outer branch of tentacles broad, conoid; inner branch of tentacles longer than outer, tendril-like, tapering distally. Ventral sucker in anterior third of body, round, smaller than oral sucker. Prepharynx short, distinct, sigmoid or looped. Pharynx ellipsoidal to dolioform, in line with oral sucker or rotated up to 90°. Oesophagus short, bifurcates just posterior to pharynx with proximal section reaching to ventral surface and opening as ‘ventral anus’, and distal portion expanding to form caecum. Caecum single, broadest in anterior region, passes from midforebody to close to posterior extremity, terminates blindly; gastrodermis well developed.
Testes two, ellipsoidal, tandem, contiguous or separated, in mid hindbody. Vasa deferentia narrow, passing relatively direct from testes to cirrus-sac. Cirrus-sac elongate, cylindrical, gently winding, reaching from just anterior to ovary to posterior margin of ventral sucker. Internal seminal vesicle tubular, loops once about mid-length, occupies less than half length of cirrus-sac. Pars prostatica distinct, vesicular, less than half length of internal seminal vesicle, lined with anuclear cell-like bodies. Ejaculatory duct distinct, approximately equal in length to, or longer than, pars prostatica; opens into genital atrium. Genital atrium narrow, posterior-dorsal and subequal to ventral sucker. Genital pore small, round to irregular, opening dorsally at level of ventral sucker.
Ovary pre-testicular, pyriform, narrowing posteriorly toward union with oötype. Mehlis’ gland indistinct. Laurer’s canal opens dorsally at level of ovary. Canalicular seminal vesicle saccular, contiguous with and dorsal to ovary. Uterus narrow, passes posteriorly from oötype to anterior testis, loops back, gently winding anteriorly, forming muscular metraterm about mid-level of pars prostatica, opening into genital atrium adjacent to ejaculatory duct. Eggs few, oval, operculate, large; length often exceeding that of ovary. Vitellarium follicular, restricted to hindbody; fields reaching from about mid-cirrus-sac to near posterior extremity; dorsal, lateral and ventral fields confluent, wrap around body from dorsal midline to ventrosinistral and ventrodextral regions anterior to testes, wrap entire body posterior to testes. Vitelline reservoir between ovary and anterior testis; collecting ducts indistinct. Excretory pore terminal; excretory vesicle Y-shaped, passes anteriorly, bifurcating in testicular region, ducts passing anteriorly sinistrally and dextrally, terminating as enlarged pyriform sacs on either side of cirrus-sac.
Description of redia ( Fig 13C View Figure 13 ): Measurements in Table 6. Description based on voucher material. Body elongate, broadest anteriorly, tapering posteriorly. Cercarial embryos numerous, poorly developed. Mouth terminal. Pharynx dolioform. Intestine short, globular, immediately posterior and subequal in size to pharynx.
Description of cercaria ( Fig 13E View Figure 13 ): Measurements in Table 6. Description based on voucher material. Oculate gymnocephalous cercariae. Body elongate, fusiform. Eyespots two, in anterior forebody; additional pigment conspicuous, dispersed in forebody. Oral sucker terminal, infundibuliform. Ventral sucker post-equatorial, round. Prepharynx short, passes between eyespots. Pharynx ellipsoidal. Caecum single, terminating in region dorsal to ventral sucker. Tail longer than body, bipartite; proximal portion bearing series of lateral projections; distal portion scaled, lacking lateral projections. Excretory vesicle Y-shaped, arms extending to ventral sucker, stem extending to near posterior extremity; posterior collecting duct visible to first few scales of distal potion of tail; anterior collecting ducts not visible beyond ventral sucker. Genital primordia darkly stained, dorsal to ventral sucker.
Remarks: There are no clear morphometric features that differentiate this species from Gorgocephalus kyphosi , and these two species have overlapping host ranges at both the definitive and intermediate host level. However, the cirrus-sac can again be used for differentiation. In G. kyphosi , the ejaculatory duct is short and the internal seminal vesicle and pars prostatica each occupy about half of the cirrus-sac, whereas in G. graboides the internal seminal vesicle occupies less than half of the cirrus-sac and the pars prostatica is less than half the length of the internal seminal vesicle; the remaining space is occupied by a long, distinct ejaculatory duct, which is about as long as, or longer than, the pars prostatica. Furthermore, the genital atrium is larger than the ventral sucker in specimens of G. kyphosi , whereas in G. graboides the two features are similar in size.
From Gorgocephalus euryaleae , G. graboides differs in having a shorter pars prostatica (occupying less than half, rather than more than half, of the cirrus-sac) and in having a longer ejaculatory duct (equal or greater in length than the pars prostatica vs. shorter than the pars prostatica). Although both species share K. cinerascens as a host, they appear to be geographically isolated from one another.
From Gorgocephalus manteri , G. graboides differs primarily in having a larger body and in having vitelline follicles restricted to the hindbody, rather than having them extend into the forebody. The cirrus-sac of G. graboides also lacks the strong sigmoidal shape of that of G. manteri and based on the calculations of Bray & Cribb (2005), G. manteri has, as a percentage of body length, a much greater body width (28–34% vs. 8–11%), lesser ovary to ventral sucker distance (~5% vs. 27–33%) and greater ovary to testis distance (~17% vs. 4–9%) than G. graboides .
Gorgocephalus graboides is easily distinguished from G. yaaji in having a far less dorsoventrally flattened body, and in having vitelline follicles restricted to the hindbody, rather than having them extend into the forebody. Gorgocephalus graboides also differs from G. yaaji morphometrically, namely in having, as a percentage of body length, a shorter forebody (21–26% vs. 31–42%), a shorter pharynx (3.0–4.3% vs. 6.0– 10.2%) and a longer testis to ventral sucker distance (36–44% vs. 22–32%). Gorgocephalus graboides also has a lesser pharynx length to oral sucker length ratio than G. yaaji (0.27–0.35 vs. 0.37–0.55). The inner and outer portions of the oral sucker tentacles of G. yaaji are approximately equal in length, rather than having the inner portion longer like in G. graboides and the tegument scales of G. yaaji have more posterior tendrils than those of G. graboides (up to 18 vs. up to 15). Lastly, the cirrus-sac of G. graboides is less robust than that of G. yaaji and does not have the anterior portion recurved.
Gorgocephalus kyphosi View in CoL and G. graboides View in CoL share an intermediate host, Echinolittorina vidua View in CoL , at Lizard Island, GBR, and other than the cercariae of G. graboides View in CoL having a somewhat more fusiform body shape, we were unable to find any clear morphometric or qualitative differences between the intramolluscan stages of these two species. Thus, intramolluscan stages of G. kyphosi View in CoL and G. graboides View in CoL can only be distinguished on a molecular basis. The same subtle differences found between intramolluscan stages of G. kyphosi View in CoL and G. yaaji View in CoL apply when comparing G. graboides View in CoL to G. yaaji View in CoL . We obtained naturally emerged cercariae of G. graboides View in CoL and did not observe the eyespot ‘lenses’ described for naturally emerged cercariae of G. yaaji ( Huston et al., 2016) View in CoL . We also did not observe the posterior ‘protuberance’ in the rediae of G. graboides View in CoL . However, as discussed above, these seem weak characters for species delineation.
| QM |
Queensland Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Digenea |
|
Order |
|
|
SuperFamily |
Lepocreadioidea |
|
Family |
|
|
Genus |
Gorgocephalus graboides
| Huston, Daniel C., Cutmore, Scott C., Miller, Terrence L., Sasal, Pierre, Smit, Nico J. & Cribb, Thomas H. 2021 |
G . graboides
| Huston & Cutmore & Miller & Sasal & Smit & Cribb 2021 |
G . graboides
| Huston & Cutmore & Miller & Sasal & Smit & Cribb 2021 |
G . graboides
| Huston & Cutmore & Miller & Sasal & Smit & Cribb 2021 |
G . graboides
| Huston & Cutmore & Miller & Sasal & Smit & Cribb 2021 |
G . graboides
| Huston & Cutmore & Miller & Sasal & Smit & Cribb 2021 |
G . graboides
| Huston & Cutmore & Miller & Sasal & Smit & Cribb 2021 |
G . yaaji
| Bray & Cribb 2005 |
G . yaaji
| Bray & Cribb 2005 |
Gorgocephalus kyphosi
| Manter 1966 |
G . kyphosi
| Manter 1966 |
G . kyphosi
| Manter 1966 |