Fergusobia camaldulensae, Davies & Giblin-Davis & Ye & Taylor & Thomas, 2012
|
publication ID |
https://doi.org/10.11646/zootaxa.3415.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03E9878B-452A-FFD5-35DB-FD0BA377F91C |
|
treatment provided by |
Felipe |
|
scientific name |
Fergusobia camaldulensae |
| status |
|
Description of Fergusobia camaldulensae n. sp. Davies
( Figs 3–12 View FIGURES 3–12 )
Measurements. Table 3.
Material examined. The description presented here is based on measurements of 24 parthenogenetic females, 24 males, and 23 infective females from axial bud (‘stem’) gall on Eucalyptus camaldulensis Dehnh. 1832 , associated with an undescribed species of Fergusonina ; from four sites in Adelaide, South Australia, Australia: Waite Arboretum, Urrbrae ( 34º 58’ S, 138º 38’ E); Glynburn Road, Kensington ( 34º55.45’S 138º39.76’E); Hazelwood Park, Hazelwood Park (34º93.5S 138º65.8’E); Stonyfell Road, Stonyfell ( 34º55.44’S 138º39.68E). Respectively collected K. A. Davies and Sean Collett, 24.viii.2001 ; K. A. Davies, 15.viii.1999 ; K. A. Davies, 23.viii.1998 ; K. A. Davies, 12.ix.2007 .
Holotype. Parthenogenetic female, with one infective female paratype and one male paratype on a slide deposited in the ANIC, Canberra, ACT, Australia, collected at Waite Arboretum, Urrbrae , Adelaide , South Australia, Australia, by K. A. Davies and Sean Collett , 24.viii.2001, data as above.
Paratypes. Vouchers deposited at the WINC, The University of Adelaide , SA, Australia , 9 parthenogenetic ♀ s, 3 pre-parasitic infective ♀ s, 6 ♂ s; slide numbers 004447-004749, 026047, and 063799; and the USDA Nematode Collection , Beltsville MD, USA .
Description. Parthenogenetic female. From axial bud (‘stem’) galls on Eucalyptus camaldulensis . Shape arcuate to open C, posterior third to half of body dorsally curved with ventral side convex; body conoid behind vulva, and may be ventrally concave behind the vulva ( Fig. 3 View FIGURES 3–12 ); usually smaller than amphimictic pre-parasitic female and males. Cuticle obscurely annulated, appears longitudinally striated when viewed with light microscope; lateral fields not seen.
Cephalic region about 70 (63–71)% diameter of body at anterior end, offset, 1–2 µm high, unstriated; with rounded outline in lateral view and with circum-oral area centrally elevated ( Fig. 6 View FIGURES 3–12 ). Stylet with conus 40–50% of total length, basal knobs well defined, 2 µm wide at base, round ( Fig. 6 View FIGURES 3–12 ). Prominent stylet musculature apparently attached to stylet at regular intervals as well as to knobs ( Fig. 6 View FIGURES 3–12 ).
Orifice of dorsal oesophageal gland ~1 µm posterior to stylet knobs. Anterior fusiform part of digestive tract length 1.9 (1.5–2.3) times diameter, or 71 (65–78)% of body diameter; lumen of tract broadens at ~80% length of dorsal oesophageal gland. Oesophageal glands enormous, occupying ~70–85% of body diameter, extending over 30–55% (mean 43%) of total body length ( Fig. 3 View FIGURES 3–12 ).
Secretory/excretory pore obscure, with non-refractile duct, secretory/excretory cell not seen. Hemizonid extending over two to four annules, immediately in front of secretory/excretory pore ( Fig. 3 View FIGURES 3–12 ).
Reproductive tract on ventral side; occasionally flexed at nerve ring growing back towards tail on dorsal side of the oesophageal gland. Variable in length, extending part-way along gland (in 8 of 15 specimens examined) or to nerve ring (in 7 of 15 specimens); occasionally with one or two flexures (in 2 of 15 specimens); oviduct with two oocytes per row; uterus ca 35% body length, non-extensile, containing no eggs (in 12 of 15 specimens) or one egg; vulva a depressed slit. Body narrowing gradually behind vulva.
Tail short, cylindroid, just dorsally arcuate (in 7 of 15 specimens) or in line with body (in 8 of 15 specimens); length 1–2× anal body diameter, tip rounded to bluntly rounded ( Fig. 9 View FIGURES 3–12 ).
Infective pre-parasitic female. From axial gall on E. camaldulensis . Infecting mature larval or pupal stage of Fergusonina sp. Arcuate shape when relaxed by heat, tail region may be more curved than rest of body (in 2 of 30 specimens examined); sub-cylindrical body with maximum diameter at mid-body length or in posterior half, barely tapering from maximum to near tail tip ( Fig. 5 View FIGURES 3–12 ). Cuticle finely annulated, longitudinal striations visible viewed with light microscope; lateral fields obscure, not seen. Large, inconspicuous, nuclei present in epidermis.
Cephalic region offset, ~1–2 µm long, 74 (68–86)% diameter of body at anterior end ( Fig. 6 View FIGURES 3–12 ). Circum-oral area flat or slightly elevated; stylet slender, weakly sclerotised with ellipsoid basal knobs higher than wide; conus 40% of total length.
Orifice of dorsal oesophageal gland about 1µm posterior to stylet knobs. Anterior fusiform part of digestive tract length 3.1 (2.5–4) times diameter, occupying 47 (42–57)% of body diameter. Oesophageal glands occupying 45–50% body diameter, extending over intestine to 30 (23– 40)% of body length ( Fig. 5 View FIGURES 3–12 ).
Secretory/excretory pore obscure, usually not seen; secretory/excretory cell not seen. Hemizonid immediately in front of pore.
Reproductive tract with uterus 60–70% of body length in uninseminated females, packed with sperm in inseminated females; vagina at right angle to body axis, plugged with refractive material; reproductive tract extending to nerve ring; hypertrophy of tract in some specimens. Vulval lips small, ~2 µm high. Tail short, length 0.8–2× diameter at anus, tip almost hemispherical ( Fig. 11 View FIGURES 3–12 ).
Parasitic female. Not seen.
Male. From axial gall on E. camaldulensis . Body arcuate to J-shaped when relaxed by heat, posterior half of body more or less curved ventrally ( Fig. 4 View FIGURES 3–12 ). Cuticle with obscure annules ~1.2 µm wide, longitudinal striations apparent when viewed with light microscope; lateral fields not seen.
Cephalic region 60–77 (mean 69)% diameter of body at anterior end, offset, 2–3 µm high, circum-oral area flat or raised, with lightly sclerotised framework; stylet 7–10 µm long, with conus 40–50% of total length, with round stylet knobs ~2 µm wide ( Fig. 7 View FIGURES 3–12 ). Anterior fusiform part of digestive tract length 2.4 (1.7–3.4) times diameter, or 52–68% of body diameter. Orifice of dorsal oesophageal gland 1–2 µm behind stylet knobs. Oesophageal glands occupy ~75–80% of body diameter, extending over intestine to average 29 (22–40)% of total body length ( Fig. 4 View FIGURES 3–12 ).
Secretory/excretory pore opening opposite nucleus of oesophageal gland; secretory/excretory cell not seen. Hemizonid extending over two or three annules, immediately in front of secretory/excretory pore ( Fig. 4 View FIGURES 3–12 ).
Reproductive tract with single testis, variable in length, usually overlapping dorsal oesophageal gland (extending to nerve ring in 2 of 25 specimens examined); sometimes reflexed at tip (in 3 of 25 specimens); testis, seminal vesicle and vas deferens clearly differentiated ( Fig. 4 View FIGURES 3–12 ). Bursa smooth, peloderan; arising 51 – 71% of total body length from posterior. Spicules paired, angular near middle, blade slender, weakly sclerotised; manubrium not offset, wider than shaft, may have angular shape at its dorsal anterior tip; opening terminal ( Fig. 12 View FIGURES 3–12 ). Inconspicuous muscles associated with cloaca. Tail length 1.5–3 times diameter at cloaca; ventrally concave, arcuate, slender in comparison to rest of body; tail tip bluntly rounded ( Fig. 10 View FIGURES 3–12 ).
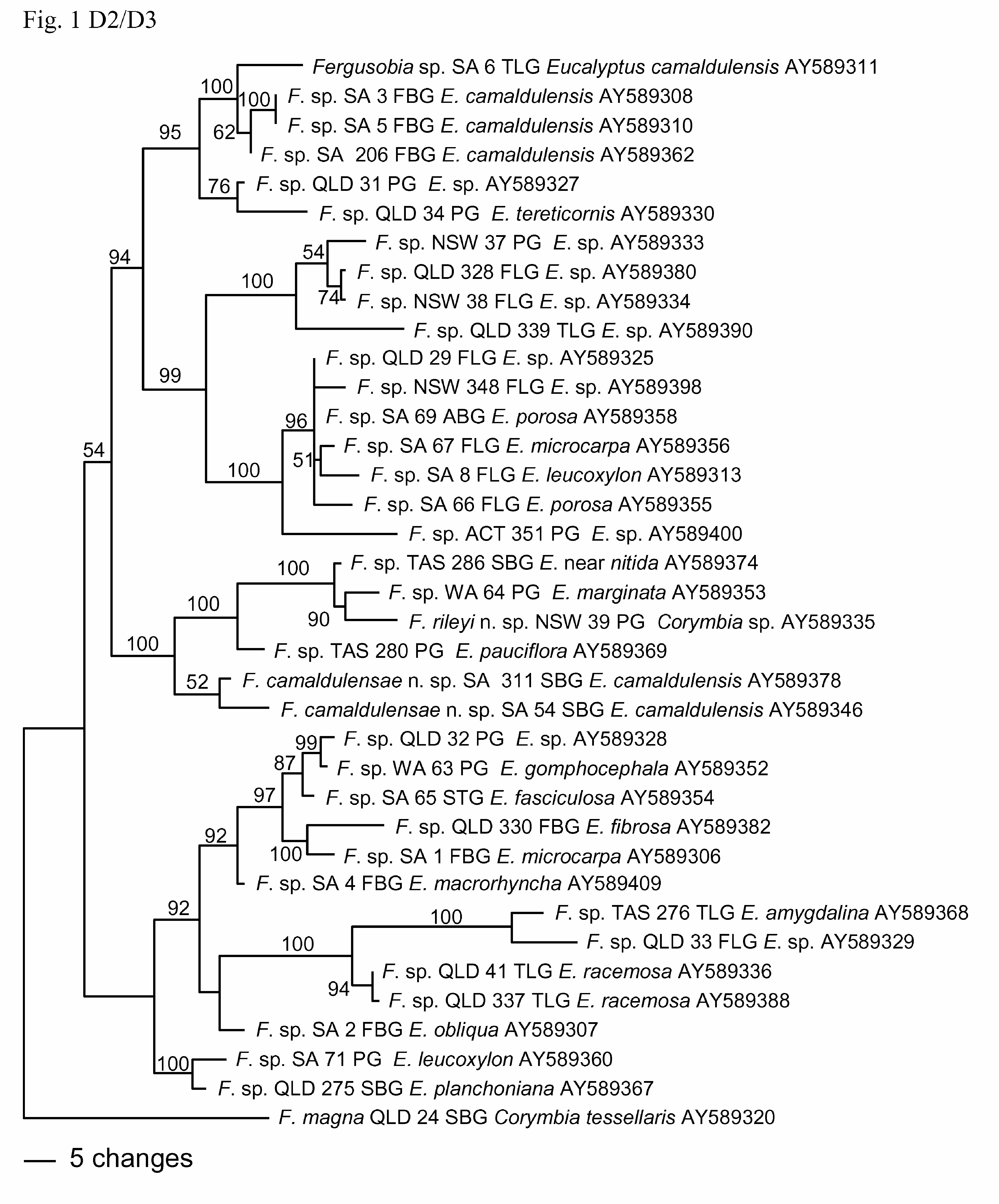
Diagnosis and relationships. Fergusobia camaldulensae n. sp. is characterized by the following combination of morphological features: an arcuate to open C-shaped parthenogenetic female with a raised circum-oral area and a broadly conoid tail; an arcuate infective female with an hemispherical tail tip; and males of varying shape with a raised circum-oral area, weakly sclerotised angular spicules and bursa arising at mid to two-thirds body length. It is also distinguished by its biogeography and Eucalyptus host range, being collected from E. camaldulensis in South Australia, associated with an undescribed species of fergusoninid fly having a dorsal shield comprising 9 ‘bars’ of sclerotised spicules, and collected from small, multilocular, nodular axial bud galls. Its status as a distinct species is corroborated by molecular data from sequencing of 28S D2/D3 and mtCOI.
In having parthenogenetic females whose body is arcuate to an open C-shape with a broadly conoid tail, F. camaldulensae n. sp. differs from F. indica ( Jairajpuri 1962) Siddiqi 1986 , F. magna Siddiqi 1986 , F. ptychocarpae Davies 2008 (in Taylor & Davies 2008) and F. brittenae Davies 2010 (in Taylor & Davies 2010b) (C or tight Cshaped after heat relaxation). In length (300–414 µm), it is apparently smaller than F. tumifaciens ( Currie 1937) Wachek 1955 (415 µm); and is larger than F. cajuputiae Davies & Giblin-Davis 2004 (221–273 µm). Length of the stylet (11–12 µm) of these parthenogenetic females separates them from F. brevicauda Siddiqi 1994 (8–8.5 µm), F. dealbatae Davies & Giblin-Davis 2004 (9–10 µm), F. fisheri Davies & Lloyd 1996 (9–10 µm), F. jambophila Siddiqi 1986 (8–10.5 µm), F. leucadendrae Davies & Giblin-Davis 2004 (7–9 µm), F. nervosae Davies & Giblin- Davis 2004 (8–9 µm), F. philippinensis Siddiqi 1994 (7.5–9 µm), F. quinquenerviae Davies & Giblin-Davis 2004 (8–10.5 µm), and F. viridiflorae Davies & Giblin-Davis 2004 (7–9 µm). Tail shape (cylindroid, more or less arcuate with bluntly rounded tip) of parthenogenetic females of F. camaldulensae n. sp. differs from that of F. rileyi n. sp. (longer, straight to arcuate, more slender), F. pohutukawa Davies 2007 (conoid, straight, some with mucron), and F. curriei Fisher & Nickle 1968 (conoid, with a broader tip).
Infective females of F. camaldulensae n. sp. are straight to arcuate in shape, which separates them from F. brittenae , F. curriei and F. magna (open C-shape); from F. ptychocarpae (strongly curved in posterior region); and from F. rileyi n. sp. (almost straight in shape). Shape of the tail tip (hemispherical) differs from that of F. philippinensis (truncate), F. rileyi n. sp. (bluntly rounded), and F. quinquenerviae , F. cajuputiae , F. dealbaltae , F. leucadendrae , F. nervosa , and F. viridiflorae (broadly rounded). Infective females of F. camaldulensae n. sp. have a raised circum-oral area lacking in F. fisheri . Infective females of F. indica , F. jambophila , F. pohutukawa and F. tumifaciens are unknown, and those of F. brevicauda cannot be separated from F. camaldulensae n. sp.
The shape (arcuate to just J) of the males of F. camaldulensae n. sp. separates it from F. curriei and F. ptychocarpae (J-shape); and from F. jambophila (almost straight in shape). The males are longer (368–486 µm) than F. cajuputiae (286–364 µm), F. nervosae (277–312 µm), and F. quinquenerviae (256–329 µm). Tail shape (with bluntly rounded tip) differs from that in F. magna and F. rileyi n. sp. (more slender); and F. philippinensis Siddiqi (truncate tip). The length (39–55 µm) of the tail of male F. camaldulensae n. sp. is shorter than in F. rileyi n. sp. (58–70 µm). Length of the spicule (18–27 µm) is longer than in F. leucadendrae (14–17 µm). In having an angular shape, the spicule differs from that of F. pohutukawa , where it is arcuate. In having a bursa arising at 50–70% of body length, the males differ from F. viridiflorae (bursa arising near excretory/secretory pore); and from F. brevicauda , F. dealbatae , F. fisheri , F. brittenae , and F. tumifaciens (respectively, bursa arising at about 33% of body length ( Siddiqi 1994); at 30–50% ( Davies & Giblin-Davis 2004); at about 20% ( Davies & Lloyd 1996); at about 15–20% ( Taylor & Davies 2010); and at about 35% ( Currie 1937)). Form of the tail tip separates F. camaldulensae n. sp. and F. ptychocarpae (broadly vs bluntly rounded).
In morphological appearance, parthenogenetic females of F. camaldulensae n. sp. are similar to F. rileyi n. sp. collected from leaf pea galls on Corymbia sp. , MSp 5 from flower bud galls on E. eugenioides Sieber ex Sprengel 1827 and MSp 41 from leaf pea galls on E. pauciflora ( Figs 13, 19 View FIGURES 13–20 ). Infective females are morphologically similar to those of F. fisheri , and MSp 7 and 8 from terminal shoot bud galls E. delegatensis R. Baker 1900 and E. diversifolia Bonpl. 1814 (K.A. Davies personal observation). The males are similar to those of F. ptychocarpae from Corymbia ptychocarpa (F. Muell.) K.D. Hill & L.A.S. Johnson 1995 and MSp 4 from flower bud galls on Eucalyptus macrorhyncha F. Muell. 1853 (K.A. Davies personal observation).
Fergusobia camaldulensae n. sp. is also morphologically similar to an undescribed species collected from similar formless axial bud galls on E. camaldulensis , from South Australia and from Victoria, but associated with a fergusoninid fly with larvae having a shield with three confluent plates and forwardly projecting teeth (GS Taylor personal observation). The two species of Fergusobia can be morphologically separated by the length of the bursa in males (arising at the secretory/excretory pore in the undescribed species, i.e., longer); and by the shape of the tail tip (broadly rounded in the undescribed species rather than hemispherical) in the infective females. The undescribed species has not been sequenced.
From phylogenetic analyses based on sequences of D2/D3 ( Fig. 1 View FIGURE 1 ), there is strong (100%) support for inclusion of F. camaldulensae n. sp. within a clade including nematodes from shoot bud galls on E. sp. near nitida, and leaf pea galls on E. marginata , E. pauciflora and Corymbia sp. ( F. rileyi n. sp.), but its position within the clade is poorly supported (52%). This is similar to the groupings found in the trees in Ye et al. (2007) and Davies et al. (2010a).
Etymology. Named for E. camaldulensis , the host plant species from which the nematodes were collected.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.