Dicranoses capsulifex Kieffer and Jörgensen, 1910
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3682.2.9 |
|
publication LSID |
lsid:zoobank.org:pub:FCCE5B34-7DEB-4B8E-B111-35501EC37A1C |
|
DOI |
https://doi.org/10.5281/zenodo.6156025 |
|
persistent identifier |
https://treatment.plazi.org/id/405E883B-FFAB-704D-FF36-89195E7EF874 |
|
treatment provided by |
Plazi |
|
scientific name |
Dicranoses capsulifex Kieffer and Jörgensen, 1910 |
| status |
|
Dicranoses capsulifex Kieffer and Jörgensen, 1910 View in CoL
Dicranoses capsulifex Kieffer and Jörgensen, 1910: 385 View in CoL –386, figs. 14–16; Dalla– Torre, 1913: 1290 (diagnosis); Jörgensen, 1917: 5, pl. 1, fig. 13 (diagnosis); Houard, 1933: 207, figs. 472, 473 (key to identify cecidogenic insects); Mani, 1964: 181 (diagnosis); Sattler, 1973: 192 (species nomenclature); Davis, 1984: 18 (Neotropical checklist); 1998: 79–80 (family diagnosis); Hoare and Dugdale, 2003: 55 ( Cecidosidae View in CoL world checklist); Maia, 2006: 18 (list of Lepidoptera View in CoL galls for Central and South America).
Type material. Type (s): Chacras de Coria and Mendoza town plazas. Type (s) lost.
Diagnosis. Adults of D. capsulifex can be differentiated from other Neotropical Cecidosidae (except Dicranoses congregatella Bréthes ) by the absence of mouthparts and epiphysis, reduced wing venation, absence of pectinifer and presence of small, sclerotized dentations on the posterior dorsal margin of the male valva, and the presence of anterior internal dorsal crest in the female genitalia. The pupa is characterized by a cephalic process (gall cutter) bearing seven spines; posterior margin of tergum A8 with two central, horn-like acute processes; and abdominal segments A2–8 with a transverse band of spines arranged in approximately three rows at the center of the terga. The larva is distinguished by well-marked, slender, and slightly divergent hypostomal ridges, joined posteriorly as an U-shaped structure; and absence of tubular spinneret, setae, and stemmata. The gall is similar to a Schinus leaf, and is cylindrical, elongated; with the initiation of pupation, a light brownish band is differentiated close to the end of the gall and forms the “hood; the pupa pushes apart the “hood” for adult emergence.
D. capsulifex and D. congregatella share all the characters listed above. The major differences between the two species are: male genitalia with apex of cucullus more extended ventrally and the vinculum more broadly triangular in D. congregatella . Galls of D. congregatella grow inside the stem and the bark is ruptured at the final stage, the galls are cylindrical with a blunt tip, with walls thicker than those of D. capsulifex , and they are grouped in a cluster (i.e., gregarious). When the bark ruptures, more than 40 galls grouped adjacent to each other are exposed.
Adults. Male. Head. Sparsely covered by hair-like scales; mouthparts absent; eyes large (interocular index ( Davis 1975) = 0.965± 0.093 mm, n = 4) and located low on the head capsule ( Fig. 5 View FIGURES 1 – 7 ). Antenna filiform, covered by black spatulate scales, between 0.53–0.64X as long as forewing, flagellomeres covered by elongate sensilla.
Thorax. Anterior arms of laterocervical sclerites long and slender, expanded posteriorly. Metafurca with elongated, slender, postero-dorsal apophyses free from secondary arms; antero-dorsal apodemes absent ( Figs. 3, 4 View FIGURES 1 – 7 ). Sparsely covered with hair-like scales; patagiae absent; tegulae reduced, subquadrate, with a small posterior-dorsal projection that covers a minute anterior portion of forewing base. Legs with tibial spurs 0–2–4; epiphysis absent; tibial length proportion (anterior / middle / posterior legs) ~ 0.7/0.8/1.0; all pretarsi without claws; arolium well developed with numerous, minute ventral spines ( Figs. 6, 7 View FIGURES 1 – 7 ). Wings densely covered with long hair-like scales; scales along dorsal margin as long as wing width. Forewing length: 3.7–4.0 mm; venation ( Fig. 2 View FIGURES 1 – 7 ): Sc ending near midpoint of wing margin; radius with two branches, R and Rs; media with either one branch or sometimes a short second branch, when two branches, M1 separating near apex; CuA two branched, CuP faint and not stalked with 1A+2A. Hindwing length 0.75X as forewing length; venation ( Fig. 2 View FIGURES 1 – 7 ) with Sc+R ending at basal third of costal margin, Rs unbranched, nearly straight; medial vein absent or as single branch from Cu; CuA two branched, CuP absent; 1A+2A less than half of wing length, not extending to wing margin. Abdomen. Densely covered with hairlike scales; first tergum subtriangular, with blunt anterior end and sclerotized slender anterior apodemes laterally; second tergum sclerotized as a central rectangle with anterior margin projected laterally as two slender bands joined with first tergal bands; second sternum with subquadrangular lateral areas, each one projected anteriorly as a slender line. Tergosternal connection absent. Other terga sclerotized as longitudinal subrectangular central areas and sterna as transverse subrectangular areas, with lateral margins surrounding a spiracle. Genitalia ( Figs. 8–13 View FIGURES 8 – 13 ). Uncus consisting of a pair of oval, wrinkled, setigerous lobes, subtriangularly elongated posteriorly; socii absent. Transtilla extended, with dorsal portion oval. Juxta less than 0.5X as long as aedeagus, oval, very slender along anterior half ( Fig. 12 View FIGURES 8 – 13 ). Valva broad at base, reduced to half its wide on distal third, then expanding, with two or three sclerotized small, subapical dentations on ventral margin ( Fig. 9 View FIGURES 8 – 13 ); hair-like scales present on posterior half and scobinated on anterior half to the costal margin ( Figs. 10, 11 View FIGURES 8 – 13 ); pectinifer absent; costa sclerotized, 0.5X as long as valva. Vinculum relatively slender, Y-shaped. Aedeagus ( Fig. 13 View FIGURES 8 – 13 ) long and slender, anterior end widened; vesica without cornuti.
Female ( Fig. 1 View FIGURES 1 – 7 ). Forewing length: 2.7–3.5 mm; interocular index = 1.65± 0.35 mm, n = 4. Similar to male; abdominal segment A7 ~ 3x the length of A6; caudal margin bearing a dense ring of stout, elongate setae; ventrocaudal margin with a prominent, setose lobe with minutely serrated margins ( Fig. 17 View FIGURES 14 – 17 ). Genitalia ( Figs. 14–17 View FIGURES 14 – 17 ). Oviscapt cone (“functional unit formed by the hindmost segment/s visible in the resting moth and from which the anterior apophyses arise, consisting of segment VIII and sometimes segment IX” according to Kristensen, 2003), present ( Fig. 16 View FIGURES 14 – 17 ), with anterior internal dorsal crest present ( Fig. 14 View FIGURES 14 – 17 ). Anterior apophyses long, extending beyond abdominal segment 5; posterior apophyses ~ 1.5x length of anterior apophyses; apex fused to form an acute “cutting blade”, with minute serrations along both dorsal and ventral edges ( Fig. 15 View FIGURES 14 – 17 ). Genital chamber less than 1/ 4x as long as segment A7. Spermatheca as long as corpus bursae, without lateral lagena; caudal part of ductus spermatheca sometimes coiled. Ductus and corpus bursae membranous; signa absent.
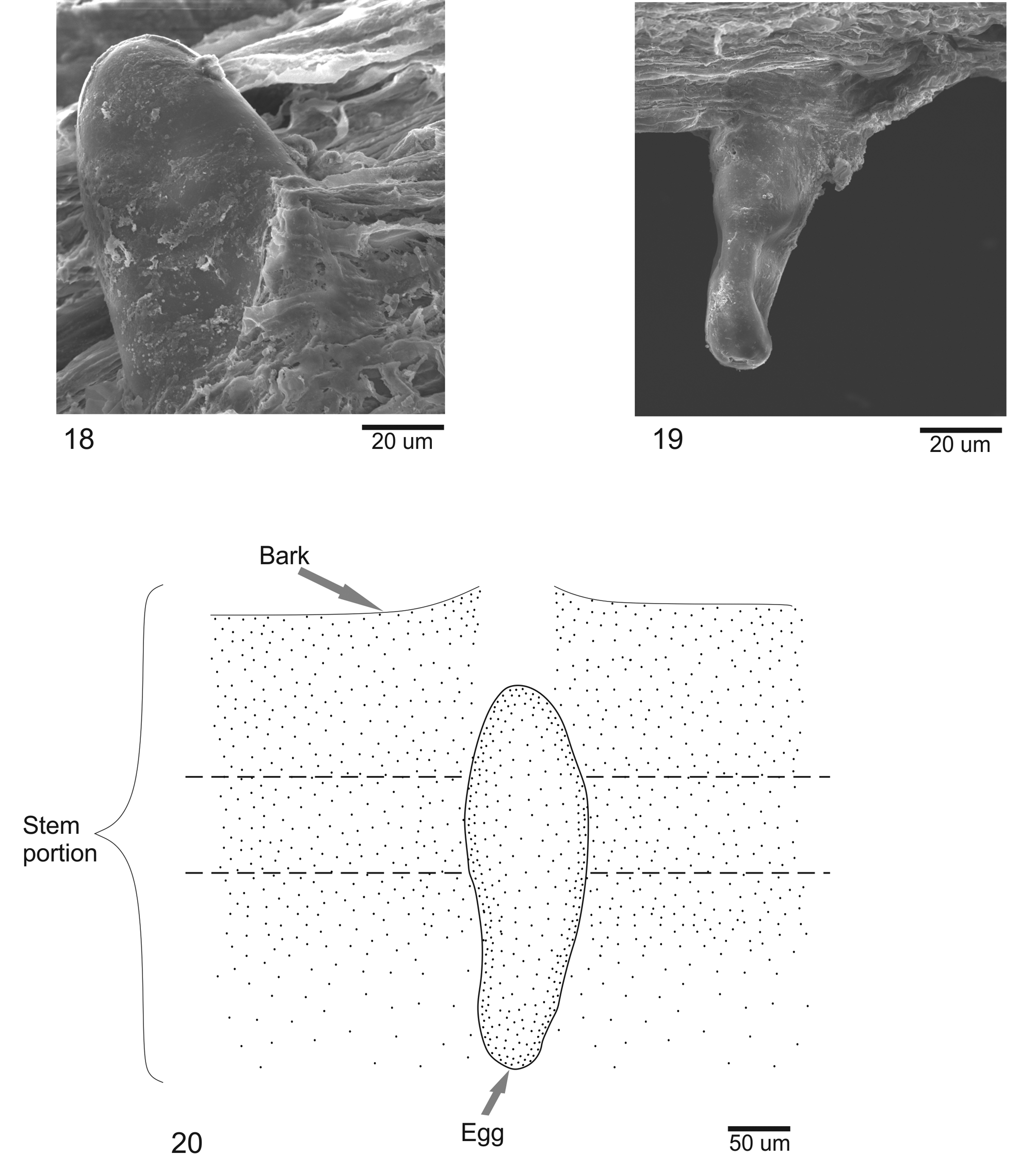
Immature stages. Egg ( Figs. 18–20 View FIGURES 18 – 20 ). Cylindrical, white, smooth, length 0.31± 0.07 mm and major diamete 0.11± 0.03 mm (n = 5).
First instar larva ( Figs. 21–23 View FIGURES 21 – 30 ). Body cylindrical, U-shaped, minute spinules and setae absent ( Fig. 21 View FIGURES 21 – 30 ). Head yellowish brown, width = 0.029± 0.005 mm, n = 4, ~ 1.3x as broad as high, with convex lateral margin, edges well defined by a sclerotized margin; frontoclypeus minute, with well-marked, slender, slightly divergent hypostomal ridges, joined posteriorly as an U-shaped structure. Stemmata absent; antennae reduced, located close to mandibles; labrum reduced to two lateral lobes; mandible well developed with four cusps along distal margin; maxilla with palpus and galea poorly developed; spinneret reduced to a central opening; labial palpus absent ( Figs. 22, 23 View FIGURES 21 – 30 ). Thorax and abdomen white; prothoracic shield, thoracic legs, prolegs, and abdominal calli absent; posterior dorsal half of abdomen with numerous small, dark brown spots.
Second instar larva. Similar to fourth instar. Head width = 0.113± 0.009 mm, n = 20.
Third instar larva. Similar to fourth instar. Head width = 0.205± 0.023 mm, n = 20.
Fourth instar larva ( Figs. 24–30 View FIGURES 21 – 30 ). Subprognathous, body cylindrical, covered with minute spinules ( Fig. 24 View FIGURES 21 – 30 ). Head ( Fig. 27 View FIGURES 21 – 30 ) yellowish brown, width = 0.384± 0.029 mm, n = 20, ~ 2x broader than high, with convex lateral margin; frontoclypeus well-marked by pigmented adfrontal sutures, subtriangular in shape, posteriorly extending to apex of epicranial notch; with well-marked slightly divergent hypostomal ridges, joined posteriorly as an U-shaped structure; basistipes and postmentum areas unpigmented. Stemmata absent; antennae reduced ( Fig. 30 View FIGURES 21 – 30 ), located laterally and dorsal to the labrum; labrum bilobed, with two pairs of small setae on distal margin, setae on external surface absent; mandible well developed with four cusps along distal margin, anterior one larger than the others; three anterior cusps sharp, fourth blunt, and one small seta basally on external surface; maxilla with palpus and galea poorly developed; spinneret reduced to a central opening; labial palpus absent ( Figs 26, 29 View FIGURES 21 – 30 ). Setae absent. Thorax and abdomen white; prothoracic shield absent; meso- and metathorax with latero-dorsal small pigmented areas; without calli; thoracic legs reduced to circular, unsegmented tubercles ( Fig. 28 View FIGURES 21 – 30 ); prolegs absent. Setae small, 2– 3 X as long as minute spinules ( Fig. 25 View FIGURES 21 – 30 ), located in groups similar in number and position to those of pupae ( Fig. 24 View FIGURES 21 – 30 ): tergum T1 with two to four setae on each side; terga T2–T3 with a pair of latero-dorsal groups of four to ten setae each; segment A1 with supraspiracular setae varying between three to five; A2–7 with five to ten supraspiracular setae; three to seven subspiracular setae on segments A2–7; segments A8–10 without spiracle; segment A8 with five to seven “supraspiracular” setae and one to four “subspiracular” setae; segment A9 with three “supraspiracular” setae; segment 10 without setae; spiracles circular.
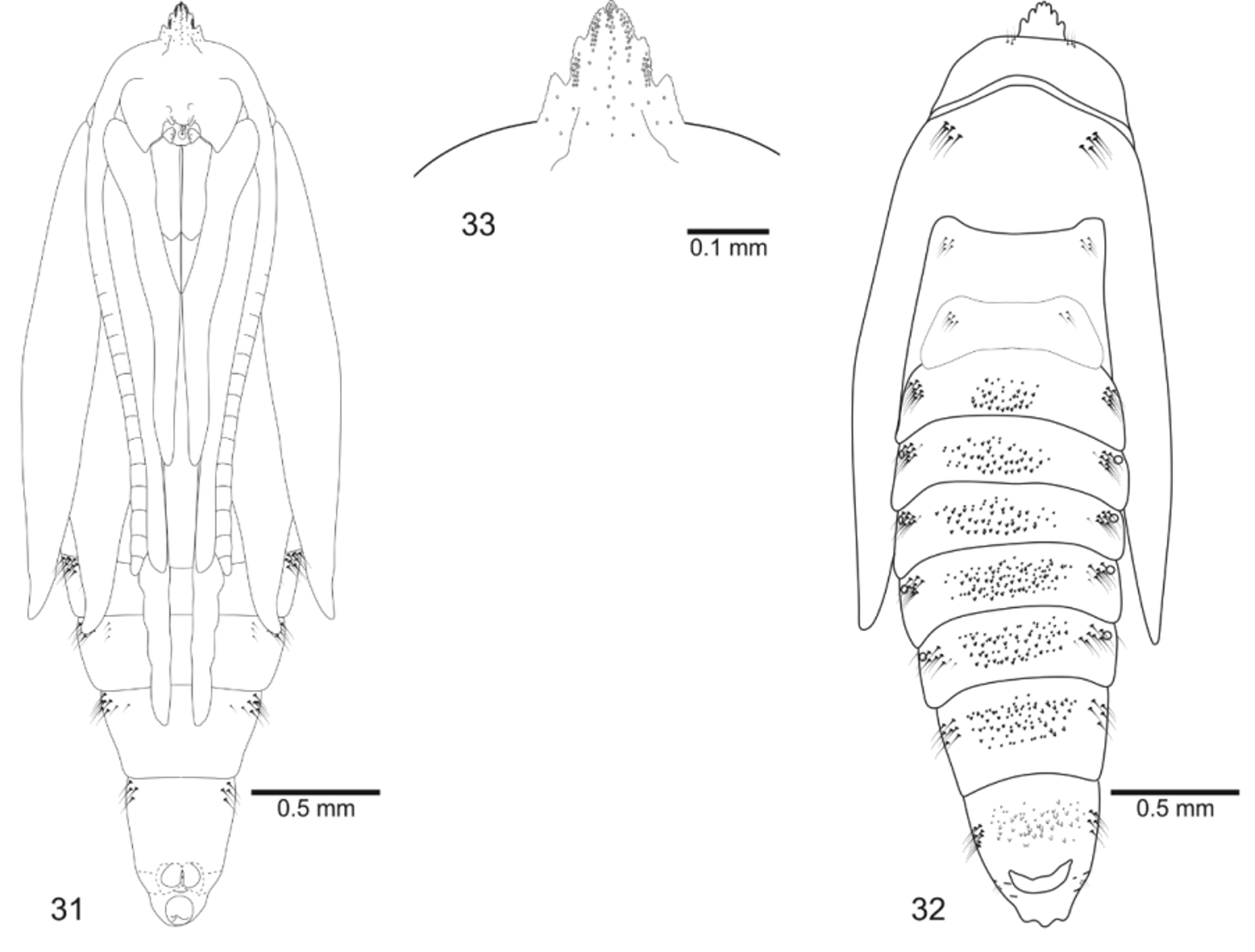
Pupa ( Figs. 31–33 View FIGURES 31 – 33 ). Length = 3.38±0.31, n = 10. Yellowish brown with legs, wings, and antennae white; colored areas becoming dark brown near adult emergence. Head with frontal process (gall-cutter) formed by seven spines in a single row, three apical ones smaller than the others ( Fig. 33 View FIGURES 31 – 33 ); antennae narrow, long, apex near apex of forewing; prothorax a narrow transverse band between head and mesothorax; hindwings concealed by forewings, reaching sternum A6; mesothoracic legs reaching forewing apex and metathoracic legs beyond them on segment A7 ( Fig. 31 View FIGURES 31 – 33 ); tergum T1 with two to four setae on each side; terga T2–T3 with a pair of latero-dorsal groups of four to ten setae each. Abdominal segments covered by minute spinules; A2–8 with a transverse band of spines arranged in approximately 3–4 rows near center of terga ( Fig. 32 View FIGURES 31 – 33 ), A2–A7 with sharp spines, A8 with spines possessing irregular margins, sometimes bifurcated; cremaster at posterior margin of tergum A8 with two central, horn-like, acute processes directed anteriorly ( Fig. 32 View FIGURES 31 – 33 ). Setae ( Figs. 31, 32 View FIGURES 31 – 33 ) arranged in two pairs of groups on each segment (supra- and subspiracular); supraspiracular setae varying between three to five on segment A1 and five to ten on segments A2–7; one to five subspiracular setae on segment A2–7; segments A8–10 without spiracle; segment A8 with six to seven “supraspiracular” setae and one to three “subspiracular” setae; segment A9 with three “supraspiracular” setae; segment 10 without seta; spiracles circular.
Gall. The galls are located on terminal branches between leaves. Initially the galls are spherical and completely enclosed on stems. Later, the galls rupture the bark and appear as small, reddish-brown prominences, increasing in size as cylindrical structures gradually turning green, thus mimicking leaves ( Fig. 36 View FIGURES 34 – 39 ). With the advent of pupation, a narrow, light brownish band appears at the base of the conical top of the gall, or “hood. This band is the site where the gall will break when the pupa emerges ( Fig. 37 View FIGURES 34 – 39 ). Utilizing the frontal process, the pupa opens the gall by pulling back the hood, and with body contortions and anchoring its body to the gall with its abdominal spines, the pupa partially exits the gall and the adult emerges. Generally, after emergence, the head and thorax of the pupa is found protruding from the apical part of gall with the hood attached laterally.
Gall size after adult emergence: length 11.772± 4.619 mm and diameter 1.225±0.207, the apical 1.8±0.302 constitutes the hood (n = 20).
On heavily infested branches, galls from the apical part of the branches develop less than the lower galls. Usually, the ones at the apex die or require more time to develop and are smaller. The largest number of galls per branch was found at Chacras de Coria, Mendoza, Argentina, with up to 110 galls on 5 cm long branches.
Host plant. In Mendoza, Argentina, D. capsulifex was found on Schinus fasciculatus ( Fig. 34 View FIGURES 34 – 39 ); but in other provinces in Argentina (e.g., San Luis) it was found to occur on S. fasciculatus and S. johnstonii Barkley (identification according to Steibel & Troiani 2008). Authors have cited D. capsulifex on Schinus polygamus or Duvana (= Schinus ) dependens. Some subspecies or varieties of these species are synonyms of S. fasciculatus . The current classification of the genus Schinus is complex and controversial, thus, the identity of the plants cited here should be taken with caution.
Distribution. This species occurs in the central and north-western provinces of Argentina.
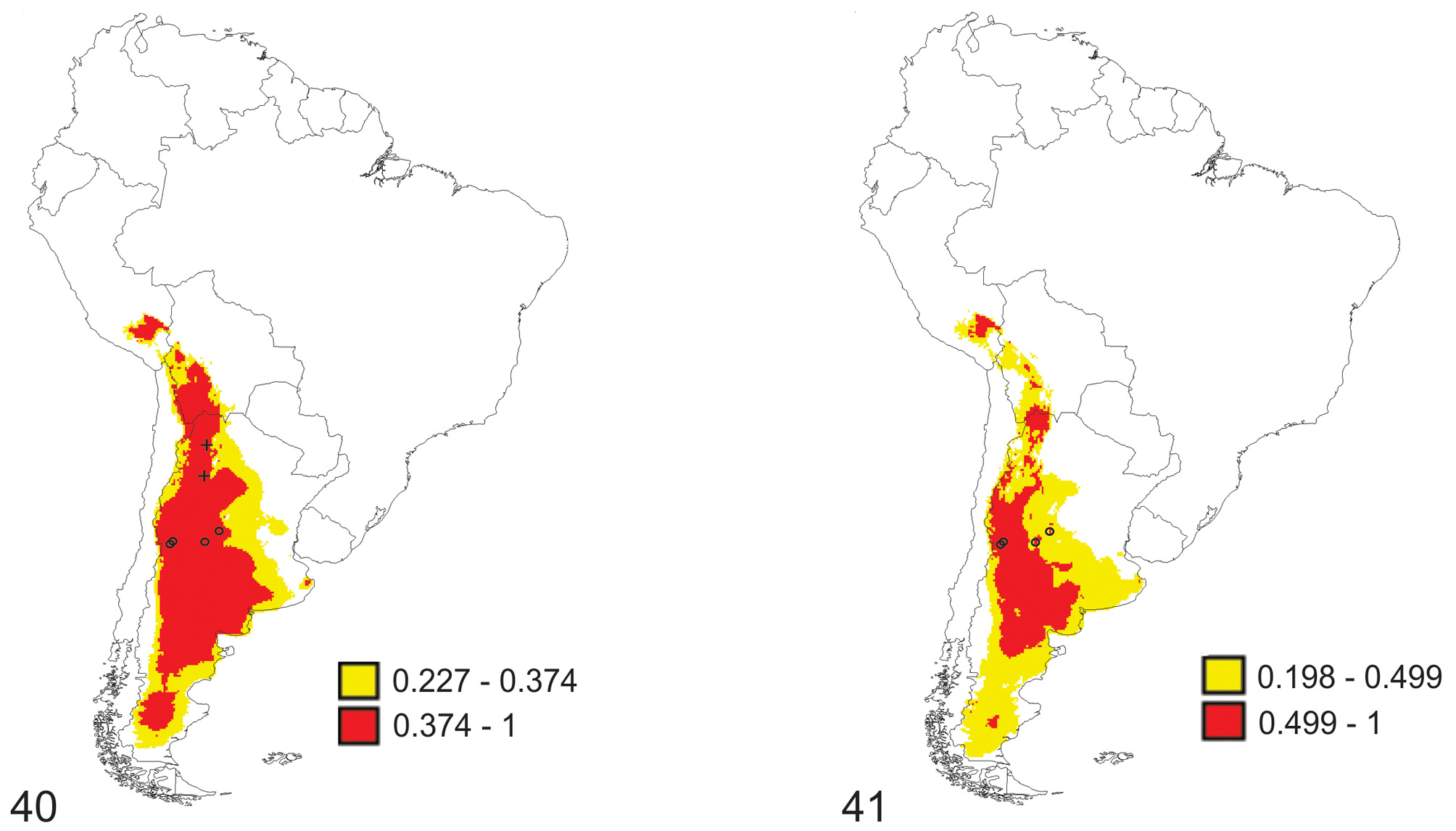
Environmental Niche Models ( Figs. 40, 41 View FIGURES 40 – 41 ) show a larger distribution than the one observed. In both analyses, with ( Fig. 40 View FIGURES 40 – 41 ) and without bibliographical data ( Fig. 41 View FIGURES 40 – 41 ), the bioclimatic variables that most contribute to ENM’s are: Temperature Seasonality (BIO4) and Temperature Annual Range (BIO7). Both variables together explain more or less 73% of the species distribution.
Life history. The female lays eggs from October to November. A few days after oviposition, first instar larvae hatch and can be found in a C-shaped position in areas of softer dark green tissue inside stems. Larvae remain in the first instar until May, when second instar larvae can be found. By the second instar, the gall is composed of harder tissue, and becomes spherical in shape as it starts to grow. Later, a small cylindrical, reddish brown part of the gall appears through the bark; this cylindrical part is the one that grows into the final gall. In July there are galls from just emerging from the bark to galls up to 10 mm in length. As the gall becomes longer, it becomes cylindrical in form, and its color changes to green, with the larva extended longitudinally inside and going to the top to feed. Although gall length more or less corresponds to the larval instar, the length cannot be used to predict with certainty the instar number ( Table 1). As larva feeds on the upper part of the gall, the inner tissues grow and obstruct the basal part of the gall. When the gall has grown to more than 7 mm in length, an internal blockage is formed and the larva becomes confined to the distal part of the gall. At the same time, the gall begins to shrink at the blocked part and resemble a rachis, with the other part of the gall resembling a cylindrical leaf. In September, approximately one month before adult emergence, the top of the gall begins to shrink and a brownish band appears. During this period pupae can be found. From the band to the top, the gall assumes a conical shape and forms the “hood”. A schematic succession of stages over time is shown in figure 42.
Instar Gall length (mm)
minimum maximum average 1 0 0 0
2 0 3,1 1.24 ± 1.45, n = 34 3 1,1 7,3 3.54 ± 1.45, n = 38 4 3,8 11,8 7.34 ± 1.52, n = 44 Adults emerge from October to November. Adults begin to emerge early in the morning after the sky begins to lighten. Before dawn adults fully extend their wings to dry and then begin to fly. They fly erratically but strongly; females usually crawl over the plant and fly less than males. Virgin females curve the abdomen dorsally and make circular movements, possibly as a pre-mating behavior and to disperse pheromones. When a male approaches, the female directs the abdomen straight to the male and the latter turns around and the pair copulate for nearly three minutes. After mating, the female does not lift the abdomen again, and 15 minutes later it flies away searching for a place to oviposit. At first a female lays eggs on green tender twigs, later she lays eggs on old branches where there were galls previously. The female strikes the twig with the top of her abdomen several times to make the hole for the egg and finally she lowers the tip of the abdomen close to the twig and deposits one egg. The female drills a hole of ~ 0.15 mm diameter and inserts the egg until almost all the egg is inside the tissue. Sometimes several eggs are left out of the hole and become desiccated and die. The plant epidermis surrounding the hole becomes necrotic and turns brown, making it easy to locate the oviposition site. After a week, the holes close, and the oviposition sites resemble short, thick brownish lines contrasting with the grayish bark ( Fig. 35 View FIGURES 34 – 39 ). When females are placed in cages with restricted oviposition sites, they may also oviposit in other parts of the plant, including the leaf rachis, midrib, and even on other galls. In instances of severe attacks, when many eggs are laid on the same twig, both the twig and the eggs may die.
Adults of both sexes become more active in sunshine, congregating in areas of the cage where it is most sunny. If the cage is taken away from the sun, all the adults retreat to the bottom, where their activity decreases significantly. Activity is restored as soon as the cage is placed in direct sunlight again. Adults die around noon of their first day of life.
Predation. Polybia scutellaris (White) ( Hymenoptera . Vespidae ) was found to prey on larvae ( Fig. 38 View FIGURES 34 – 39 ). These wasps make a longitudinal incision on the gall to remove and eat the larvae ( Fig. 39 View FIGURES 34 – 39 ). At Chacras de Coria, more galls were attacked by these wasps than those from which adults successfully emerged.
Parasitoids. Promerisus gallicola Kieffer and Jörgensen ( Hymenoptera . Chalcididae ) is a small parasitoid wasp that attacks the larvae of D. capsulifex . The adults synchronously emerge with Dicranoses capsulifex adults from a circular hole on the gall. In parasitized galls, the parasitoid larvae kill the host’s larva before it can pupate; therefore, in these galls one finds only pupae of the parasitoid.
Material examined. Adults: ARGENTINA: Mendoza. Las Heras, Blanco Encalada, 4 km SE Puesto La Crucecita (33º01’39.97’’S, 69º00’03.99’’W, 1164 m.s.l.) 1 3 2Ƥ 5–18-XI-2007 (GSB) ( USNM), 43 3Ƥ 24–31-X- 2008 (GSB) (IADIZA); Luján de Cuyo, Chacras de Coria, 300 m NE estación Paso de Los Andes (32º59’00’’ S, 68º53’14.56’’ W, 947 m. s.l.) 13 1Ƥ 1-X-2008 (GSB) ( IFML), 223 23Ƥ 11–22-X-2008 (GSB) ( USNM, USNM slides 34342, 34342, 34359, 34360, 34361, 34363, 34384), 13 17-X-2008 (GSB) (IADIZA), 1Ƥ 6-X-2009 (GSB) (IADIZA), 43 2Ƥ 8-X-2009 (GSB) (IADIZA), 13 31-X-2009 (GSB) ( IFML), 33 16-XI-2009 (GSB) (IADIZA), 13 6Ƥ 18-XI-2009 (GSB) (IADIZA), 83 4Ƥ 1–2-XI-2011 (GSB) (IADIZA), 83 1Ƥ 5–6-XI-2011 (GSB) (IADIZA), 43 4Ƥ 7–9-XI-2011 (GSB) (IADIZA).
Immature stages: ARGENTINA: Mendoza. Las Heras, Blanco Encalada, 4 km SE Puesto La Crucecita (33º01’39.97’’S, 69º00’03.99’’W, 1164 m.s.l.) 1 pupa 23-X-2007 (GSB) (IADIZA), 2 larvae 26-X-2007 (GSB) (IADIZA); Luján de Cuyo, Chacras de Coria, 300 m NE estación Paso de Los Andes (32º59’00’’ S, 68º53’14.56’’ W, 947 m. s.l.) 1 pupa 4-XI-2008 (GSB) (IADIZA), 22 larvae 21-VIII-2010 (GSB) (IADIZA), 45 larvae 16-VII- 2011 (GSB) (IADIZA), 23 larvae 29-VII-2011 (GSB) (IADIZA), 5 pupae 1-X-2011 (GSB) (IADIZA), 20 pupae 4- X-2011 (GSB) (IADIZA), 12 larvae 11-X-2011 (GSB) (IADIZA), 8 larvae 27-XI-2011 (GSB) (IADIZA), 12 larvae 17-XII-2011 (GSB) (IADIZA), 11 larvae 2-I-2012 (GSB) (IADIZA), 5 larvae 24-I-2012 (GSB) (IADIZA), 8 larvae 17-II-2012 (GSB) (IADIZA), 10 larvae 9-III-2012 (GSB) (IADIZA). San Luis. Merlo, Camino al Filo, entrada Reserva Floro-faunística (32º21'22.58''S, 64º57'35.16''O, 1185m.s.l.) 7 larvae 21-X-2011 (GSB) (IADIZA).
Galls: ARGENTINA: Mendoza. Luján de Cuyo, Las Vegas (33º00’59.04’’S, 69º16’20.10’’W, 1876 m.s.l.) 15-VIII-2008 (GSB); Las Heras, Villavicencio, Puesto Guarda Parques (32º31’24.4’’ S, 68º59’39’’ W, 1511 m.s.l.) 26-XI-2008 (GSB); Las Heras, Planta Hidroeléctrica Álvarez Condarco (33º02’34.64’’S, 69º03’17.68’’W, 1163 m.s.l.) 5-VII-10-VIII-2011 (GSB); Las Heras, Quebrada de la Cascada (32º55’09.19’’S, 69º14’31.26’’W, 1465 m.s.l.) 31-X-2010 (GSB). San Luis. Juana Koslay (33º17’09’’S, 66º15’33.93’’W, 831 m. s.l.) 29-IX-2008 (GSB), 2-I-2010 (GSB); Merlo, Camino al Filo, entrada Reserva Floro-faunística (32°21'22.58''S, 64°57'35.16''O, 1185m.s.l.) 24-IX-2008 (GSB).
Material from bibliographical resources (not examined). ARGENTINA: Catamarca. Quebrada del Río Andalgal ( Jörgensen 1917). Salta. Quebrada de las Lajas ( Jörgensen 1917).
Remarks. Kieffer and Jörgensen (1910) described six new species giving credit to Strand. Strand (1911) redescribed these species and stated that: several microlepidoptera reared from galls from Mendoza, Argentina, were presented to the Royal Zoological Museum in Berlin by Prof. Dr. J.J. Kieffer (Bitsch). In our search for type specimens of D. capsulifex , we requested any type material from: the Museum für Naturkunde (ZMHB) (Berlin, Germany), where Kieffer sent material according to Strand (1911); from Strand’s collection at Senckenberg Gesellschaft für Naturforschung (SGN) (Frankfurt, Germany); and from Jörgensen’s collection at Museo de Ciencias Naturales “Bernardino Rivadavia (MACN) (Buenos Aires, Argentina). Officials at these institutions reported that no type specimens were found. Furthermore, Gagne (1994) provided a short biography of J.J. Kieffer in which he noted that all type material of Neotropical cecidomyiids was lost. Gagne mentioned that he personally went to Bitsch (Kieffer’s birth place) and did not find any of Kieffer’s collection. Based on the information available, we believe that the type (s) is lost and we provide only the information provided by Kieffer and Jörgensen (1910) for the type locality. As Kieffer and Jörgensen (op. cit.) do not mention number of specimens used for the study, we refer to this material as type (s).
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Dicranoses capsulifex Kieffer and Jörgensen, 1910
| Blas, German San & Davis, Donald R. 2013 |
Dicranoses capsulifex Kieffer and Jörgensen, 1910 : 385
| Maia 2006: 18 |
| Hoare 2003: 55 |
| Davis 1984: 18 |
| Sattler 1973: 192 |
| Mani 1964: 181 |
| Houard 1933: 207 |
| Jorgensen 1917: 5 |
| Torre 1913: 1290 |
| Kieffer 1910: 385 |