Cyttaromyia lynnae De Jong, 2019
|
publication ID |
https://doi.org/10.26879/891 |
|
publication LSID |
lsid:zoobank.org:pub:A6C79E56-3CCC-484E-B6AF-EAEEE1695FF6 |
|
DOI |
https://doi.org/10.5281/zenodo.4335260 |
|
persistent identifier |
https://treatment.plazi.org/id/571F246B-FFA6-FFB9-1208-FA57AD06C16D |
|
treatment provided by |
Torsten |
|
scientific name |
Cyttaromyia lynnae De Jong |
| status |
sp. nov. |
Cyttaromyia lynnae De Jong , sp. nov.
Figures 5 View FIGURE 5 , 6 View FIGURE 6 zoobank.org/ D2C5FED7-813C-418A-B1B4-A35C286BD427 Etymology. The specific epithet is the Latin genitive case of the first name of the wife of the author (HDJ).
Holotype. USNM 621109 About USNM , deposited in the Department of Paleobiology , National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. Park site, Middle Fork of the Flathead River (Pinnacle, Montana, USA).
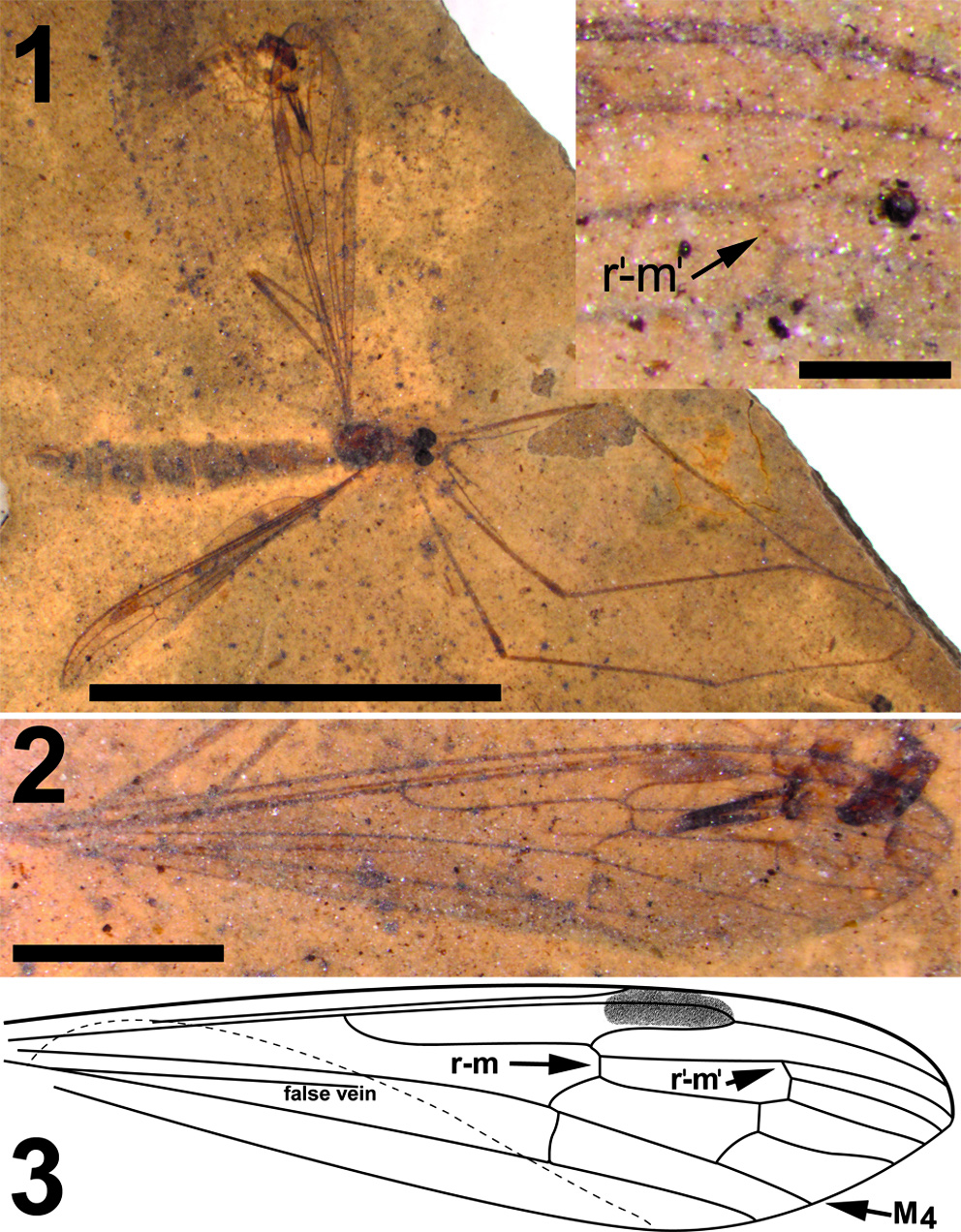
Differential diagnosis. This species of Cyttaromyia is distinguished by the presence of an additio- nal crossvein r'-m', a wide and long discal cell, four complete but relatively short medial veins, and a female terminalia with a long and curved extension of tergite 10, slender and curved cerci and broad hypogynial valves.
Description
Adult female (Figure 5.1), body length about 10 mm, wing length about 10 mm. Specimen preserved in dorsal view.
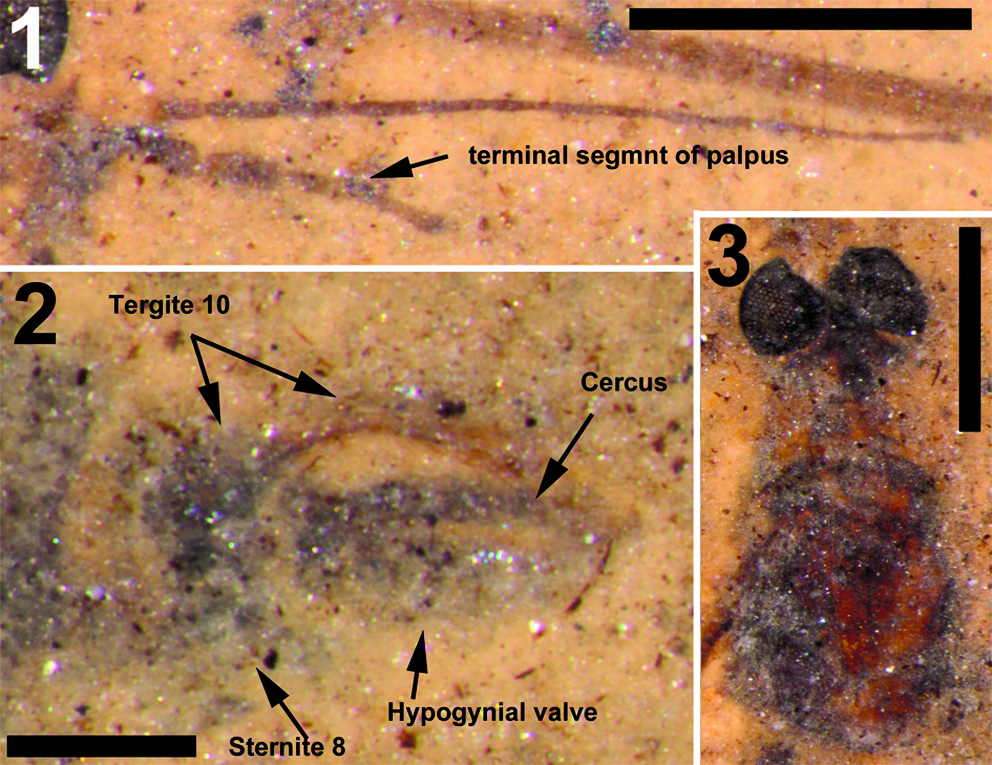
Head. Eyes well-developed, large, dorsally widely separated though distance not measurable due to crushed state of head (Figure 6.3). Occiput dark brown colored. Antenna 2.4 mm long, about as long as head and thorax combined, consisting of short scape and pedicel, and slender flagellum including 14 cylindrical flagellomeres. Some verticils at base of flagellomeres preserved, distinctly shorter than length of flagellomeres. Rostrum short. Palp with third to fifth segments visible, this part measuring about 0.9 mm, third and fourth segments robust, fifth irregularly shaped and about as long as third and fourth combined (Figure 6.1).
Thorax. Pronotum with well-developed antepronotum and broad postpronotum, scutum with visible transverse suture in posterior part. Thorax brownish in groundcolor, dark-brown on postpronotum and with broad dark-brown medial stripe and lateral sides on scutum.
Wings. Elongate and slender, anal areas folded under in both wings (Figure 5.2-3). Pterostigma distinct, located between C and first section of R 2+3+4, dark-brown. Sc long, terminating in C well beyond level of first fork of Rs and anterior to pterostigma. The positions of crossvein sc-r and R 1, if they exist, are uncertain. Rs long, almost 2.0 mm, about 0.75 X as long as entire R 2+3+4. R 5 evenly curved towards wing margin. Crossvein r-m connecting Rs with discal cell slightly distad of first fork of Rs. Vein M long, forking at proximad end of discal cell in a long first section of M 1+3 and a short first section of M 3+4. Discal cell elongate, gradually widening towards wing margin, cell remarkably longer than apical sections of M 1 -M 4. M 1+2 shortly petiolate distad of discal cell where it forks into M 1 and M 2. Base of apical part of M 1 almost perpendicular to M 2, before turning towards apex of wing; M 1 here connected to R 5 by additional crossvein (r'- m'). Apical section of M 2 almost continuous with petiole of fork of M 1+2. M 3+4 forks at distal part of discal cell, apical section of M 4 almost continuous with M 3+4 and base of M 3 making angle of about 70 o with M 3+4 and M 4. Apical section of M 3 subsinuous from discal cell towards wing margin. Crossvein m-cu located at proximad corner of dis- cal cell. CuA only very slightly bent at point of contact with crosvein m-cu; visible part of apical section of CuA straight. Remainder of venation invisible.
Legs. Front legs and left midleg almost completely preserved; what appears as right midleg with femur and part of tibia preserved. Femora somewhat broader at tip, apex darkened. Tibiae without visible apical spurs. No claws detected.
Abdomen and genitalia. Abdomen, 7.6 mm long, broadening from segment one to five and from there narrowing towards ovipositor. Ovipositor preserved in lateral view, showing long curved extension of tergite ten dorsal of cerci and hypogynial valves. Cerci long, curved and rather slender, hypogynial valves shorter than cerci and broad (Figure 6.2).
Allotype. Male unknown.
Syncompressions. Gastropods (8).
Remarks
Cylindrotomidae is a small family of Tipuloidea with only nine recent genera, including 70 species ( Oosterbroek, 2018). The recent genera occurring in North America are Cylindrotoma Macquart, 1834 (with two recent North American species), Liogma Osten Sacken, 1869 (1), Phalacrocera Schiner, 1863 (4), and Triogma Schiner, 1863 (1). The genera can be easily separated using wing venational characters ( Alexander and Byers, 1981). In a phylogenetic analysis of both molecular and morphological data, Petersen et al. (2010) recovered this group as monophyletic and sister to Tipulinae but were unable to confidently resolve the combined group within Tipuloidea; they treat the group as a subfamily within Tipulidae .
Fossil Cylindrotomidae have been described in the extant genera Cylindrotoma and Diogma Edwards, 1938 and the extinct genus Cyttaromyia Scudder, 1877 ( Table 1) ( Cockerell 1921 a, 1925, 1926; Freiwald, 1991; Freiwald and Krzemiński, 1991; Krzemiński, 1998; Podenas, 2000; Scudder, 1877, 1894; Séguy, 1934). All fossil Cylindrotomidae are characterized by the presence of four medial veins reaching the wing margin (recent Diogma always show only three medial veins). The genus Cyttaromyia was created by Scudder (1877) based on the apical half of an isolated wing. Scud- der redefined the genus in 1894 based on several more intact specimens from the Florissant Formation. Members of Cyttaromyia differ from most Cylindrotoma and Diogma (and the other recent Cylindrotomidae ) by the presence of additional crossvein r'-m'. This additional crossvein connec- ting the base of M 1 with R 5 is sometimes also found in aberrant specimens of both the North American and Palaearctic subspecies of the recent Cylindrotoma distinctissima Meigen, 1818 (cf. Brodo, 1967, fig. 46; Peus, 1952, fig. 14) and in the Eastern Palaearctic and Oriental C. taiwanica Alexander, 1929 ( Alexander, 1929, fig. 4).
Scudder (1894) described Cyttaromyia as lacking tibial spurs. This character state, in addition to the presence of r'-m', could more definitively define Cyttaromyia relative to Cylindrotoma . Howe- ver, Cyttaromyia frelloi Krzemiński, 1998 , the only specimen of the genus in Baltic amber, was described as with ”distinct tibial spurs present; single on the forelegs and midlegs, paired on hind legs. The spur of the foreleg is especially large, of a size not met till now in the Tipulidae and Limoniidae .” Tibial spurs have not been reported in the more poorly preserved compression fossils of this genus.
The structure of the ovipositor of the fossil described here (Figure 6.2) shows close similarity with that known from recent Cylindrotoma , where tergite ten is posteriorly extended into a long, curved and apically forked extension that is positioned dorsal of the cerci (cf Brodo, 1967, figs. 21, 22; Peus, 1952, figure 7b, c, 30). Krzemiński (1991a) previously suggested the similarity of the ovipositor of Cyttaromyia to that of Cylindrotoma . The species is placed in the genus Cyttaromyia because of the presence of additional crossvein r'-m', which it shares with all other species of Cyttaromyia . The crossvein can be present in aberrant specimens of some species of the extant genus Cylindrotoma .
Cyttaromyia lynnae differs from C. vahldieki Freiwald, 1991 , and C. rayona Freiwald and Krzemiński, 1991 , in not having a distinct patterning of the wings and from C. frelloi , C. quievreuxi Séguy, 1934 , and C. reclusa Cockerell, 1925 , in being female. There are numerous differences in the venation of C. lynnae compared to both C. princetoniana Scudder, 1894 , and C. fenestrate Scudder, 1877 . In the former, Rs is relatively short (Rs/R 2+3 = 1.4; 1.8 in C. lynnae ), R 1 is distinct and the distance between the 1 st fork of M and m-cu is subequal to the length of r-m whereas that value is < 0.25 in C. lynnae . The ratio of the discal cell’s L/ W = 2.4 in C. fenestrata and 3.3 in C. lynnae . In addition, Sc terminates in C in-line with the 2 nd half of the supplemental discal cell in C. fenestrata but just beyond the r-m in C. lynnae . Cells m 1 and m 2 are equal in length in C. fenestrata whereas m 2 is longer in C. lynnae . The terminus of r-r is in line with r'-m' in C. fenestrata but greatly basad of r'-m' in C. lynnae . Given the poor preservation of C. scudderi , it is difficult to identify differences with respect to C. lynnae except perhaps the shape of the pterostigma. The venation of C. obdurescens Cockerell, 1925 is also very similar to C. lynnae , although Cockerell (1926) stated that it was “similar” to C. oligocena Scudder, 1894 and “may prove a synonym of C. reclusa ”. The reliance on slight differences in venational morphology potentially diminishes the probable status of many of the fossil Cyttaromyia as separate species. Brodo (1967) has figured a large degree of intraspecific variability in the venation of multiple different specimens of the extant species Cylindrotoma distinctissima and C. tarsalis Johnson, 1912 , as well as in specimens from three additional related genera. Given the existence of numerous specimens of some of the North American species (for example, there are 12 specimens of Cyttaromyia reclusa [ Brown, 1988]), it would be of interest to study the intraspecific variability in their venation patterns.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.