Ctenochromis scatebra Genner, Ngatunga & Turner, 2022
|
publication ID |
https://doi.org/ 10.5852/ejt.2022.819.1775 |
|
publication LSID |
lsid:zoobank.org:pub:8415BF78-8949-45AA-9436-3BA3F0CAEB2B |
|
DOI |
https://doi.org/10.5281/zenodo.6560665 |
|
persistent identifier |
https://treatment.plazi.org/id/8B105DD6-6E73-4679-81D1-1A63CF668E5C |
|
taxon LSID |
lsid:zoobank.org:act:8B105DD6-6E73-4679-81D1-1A63CF668E5C |
|
treatment provided by |
Felipe |
|
scientific name |
Ctenochromis scatebra Genner, Ngatunga & Turner |
| status |
sp. nov. |
Ctenochromis scatebra Genner, Ngatunga & Turner sp. nov.
urn:lsid:zoobank.org:act:8B105DD6-6E73-4679-81D1-1A63CF668E5C
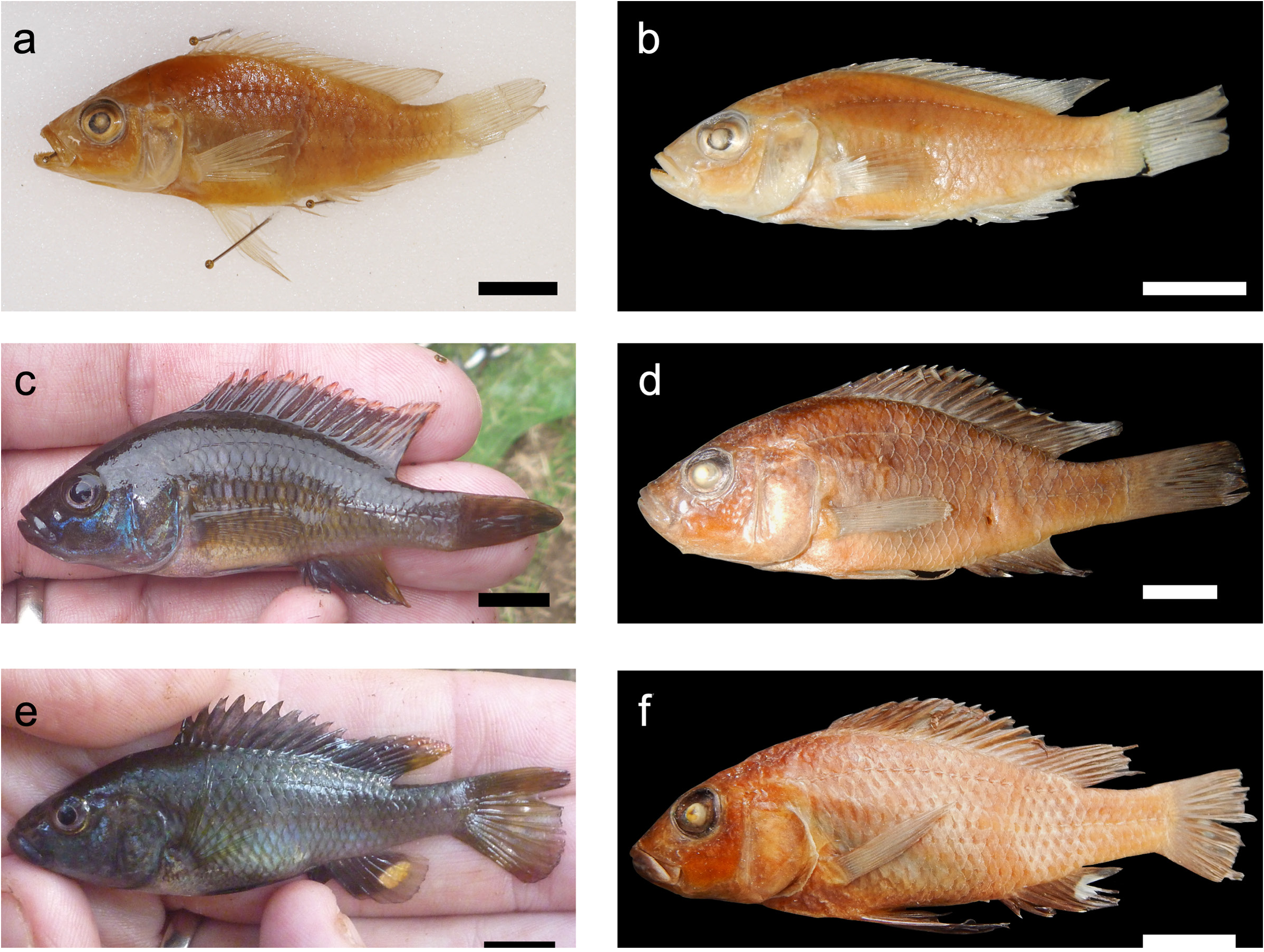
Figs 6e–f View Fig , 7g –i View Fig , 8c View Fig
Ctenochromis pectoralis View in CoL – de Graaf 2011: 38 (specimens from Chemka Springs). — van Heusden 2015: 24–27, 29 (part, specimens from Chemka Springs). — Schedel et al. 2019: 27–30. — Carleton et al. 2020: 4961, 4964, fig 2.
Ctenochromis sp. – Kalacska et al. 2017: 4 –6,18, fig. 2g –j.
Diagnosis
Ctenochromis scatebra sp. nov. is recognised as a member of Ctenochromis . This is because it possesses the diagnostic feature of a sharp break from small anterior scales to large posterior scales between the pectoral and pelvic fins, and it possesses scaleless areas on either side of the chest ( Greenwood 1979). In C. scatebra sp. nov. squamation is absent from the ventral part of the cheek, which is characteristic of the genus Ctenochromis . Mature adult male C. scatebra sp. nov. possess at least one clear non-ocellate egg spot on the anal fin.
Etymology
The species is named from the Latin noun ‛scatebra’, meaning ‛spring’ or ‛a gush of water from the ground’, referring to the type locality which is a spring in northern Tanzania.
Material examined
Holotype TANZANIA • ♂ (54.9 mm SL); Chemka Springs ; 3.443° S, 37.194° E; 17Aug. 2015; BMNH 2021.7.15.4 ( Figs 6f View Fig , 7g –i View Fig , 8c View Fig ). GoogleMaps
Paratypes TANZANIA • 9 individuals (between 33.8 and 59.1 mm SL); same collection data as for holotype; BMNH 2021.7.15.5 – BMNH 2021.7.15.13 GoogleMaps .
Description
Holotype and paratype measurements in Table 3 View Table 3 . Body laterally compressed, deeper than wide. Head (lateral view) slightly convex between eye and dorsal fin. Snout straight in lateral view, rounded in dorsal view. Mouth retrognathus. Lips slightly thickened, equally developed. Teeth in outer row primarily unicuspid, widened (shovel shaped), often slanted. Side teeth in outer row unequally bicuspid and pointed. Teeth in inner rows small, in fleshy tissue. Pectoral fin origin above dorsal fin origin, pelvic fin origin slightly more anterior. Caudal-peduncle longer than deep (caudal-peduncle depth 62.0–83.4% of caudal-peduncle length). Scales ctenoid on flanks. Scales cycloid on head, between pectoral fin and anal fin, along dorsal-fin base. Scales absent from chest. Lateral-line scales 15–21/7–11, Dorsal fin XIV–XV, 8–9, Anal fin III, 7–8.
Colour
Live colouration from images of live specimens in natural habitat (Schedel 2019). Mature males: dorsal body grey-blue, flanks lighter than dorsal with blueish sheen. Depending on mood, a very faint midline stripe and 4–5 very faint vertical bars present. Head dark grey-blue, blue sheen below and posterior to eye. Blue tinge to lower lip. Dorsal fin grey-blue with orange-red lappets, red posteriorly. Pectoral fins black. Pelvic fins with red base. Anal fin grey/blue, red posteriorly, with one or two (rarely three) non-ocellate egg spots (multiple spots tightly packed). Caudal fin light grey-blue, with red tinges at the dorsal and ventral tips. Euthanised fish: colours darker ( Fig. 6e View Fig ). Females and subadult males: flank
grey-brown base colour, white ventrally. Fins uniformly light grey-brown. Flank with 6–8 irregularly shaped and irregularly spaced vertical bars, alongside partially complete midlateral and dorsolateral stripes. Bar and stripe patterns variable among individuals, faded in some specimens (photo in van Heusden 2015). Preserved coloration: in ethanol brown or beige. Male non-ocellate egg spots on anal fin sometimes visible.
Distribution
The species is restricted to Chemka Springs and the surrounding water bodies immediately adjacent to the Springs. Water from Chemka Springs flows southwards into the Kikuletwa River towards Nyumba ya Mungu Reservoir. Surveys are needed further downstream from the site of the spring, into the river, to determine the full species distribution.
Life history
The species has been observed feeding upon epilithic and epiphytic algae in Chemka Springs, as well as sifting soft sediment (Schedel 2019), and pecking on skin of swimmers. The species is therefore most likely an omnivorous generalist. Only two other fish species are known from Chemka Springs, Garra cf. dembeensis (Rüppell, 1835) and Clarias gariepinus (Burchell, 1822) . The water maintains a steady 28.4°C ( Røhr et al. 2002).
Remarks
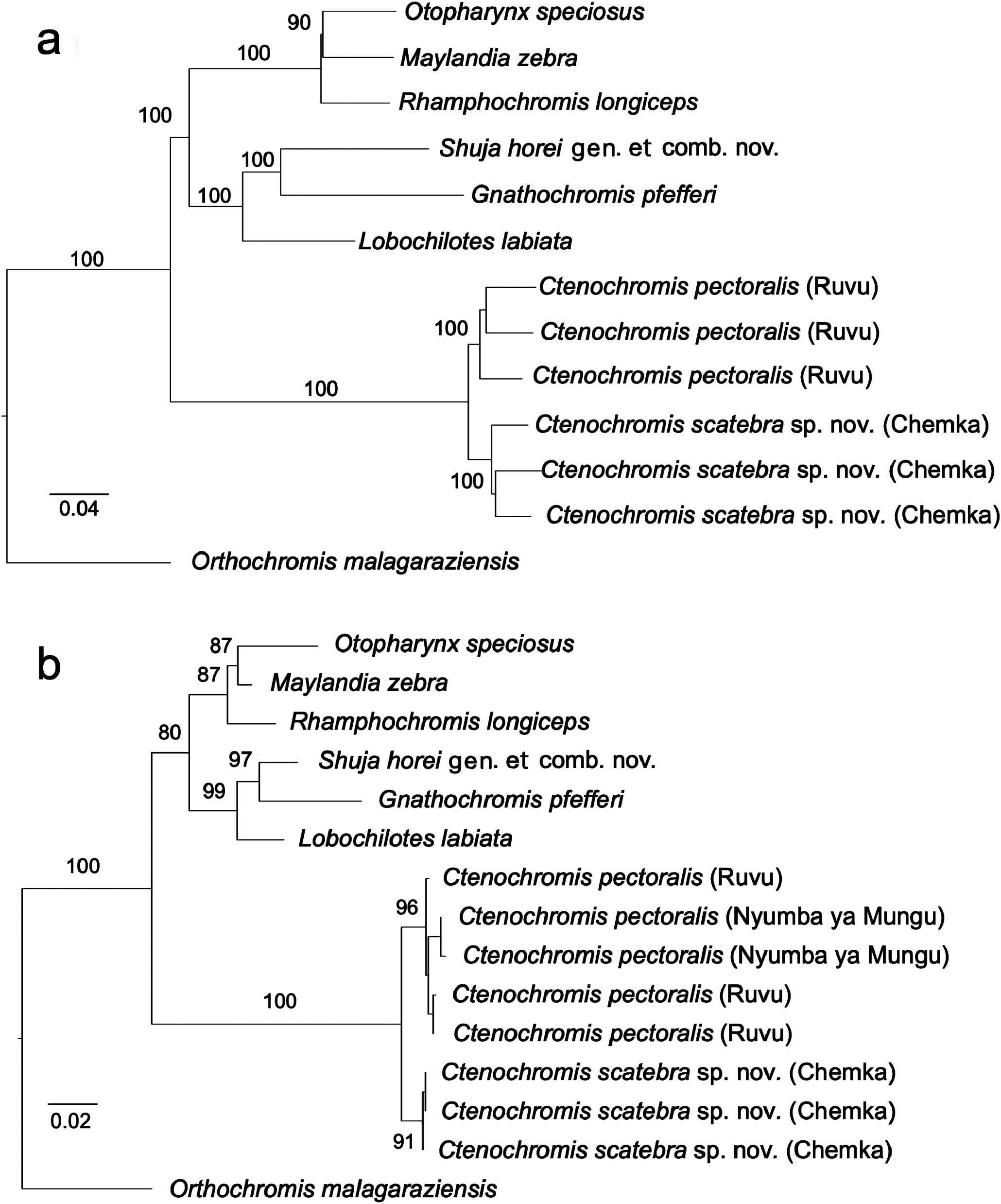
Phylogenetic analyses, based on genome-wide genetic markers, place C. scatebra sp. nov. as a sister to the type species C. pectoralis ( Fig. 4 View Fig ). Specimens of C. scatebra sp. nov. can be distinguished from C. pectoralis based on two aspects of trophic morphology: 1) C. scatebra sp. nov. has front teeth in the outer row on both jaws that are primarily unicuspid, widened (shovel shaped) and often slanted ( Fig. 7g View Fig ), while side teeth in the outer row are unequally bicuspid and pointed; by contrast all front and side teeth in the outer row of C. pectoralis are all unequally bicuspid and pointed ( Fig. 7a, d View Fig ); 2) Ctenochromis scatebra sp. nov. has a retrognathus jaw, while C. pectoralis has a marginally prognathous jaw ( Figs 6 View Fig , 8 View Fig ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Pseudocrenilabrinae |
|
Tribe |
Haplochromini |
|
Genus |
Ctenochromis scatebra Genner, Ngatunga & Turner
| Genner, Martin J., Hsu, Ling-Lan, Collins, Rupert A., Smith, Alan M., Saxon, Andrew D., Shechonge, Asilatu H., Ngatunga, Benjamin P. & Turner, George F. 2022 |
Ctenochromis sp.
| Kalacska M. & Arroyo-Mora J. P. & Lucanus O. & Kishe-Machumu M. A. 2017: 4 |
Ctenochromis pectoralis
| Carleton K. L. & Conte M. A. & Malinsky M. & Nandamuri S. P. & Sandkam B. A. & Meier J. I. & Mwaiko S. & Seehausen O. & Kocher T. D. 2020: 4961 |
| Schedel F. D. B. & Musilova Z. & Schliewen U. K. 2019: 27 |
| Van Heusden H. 2015: 24 |
| de Graaf J. 2011: 38 |