Crossodactylodes septentrionalis, Jr, Mauro Teixeira, Recoder, Renato Sousa, Amaro, Renata Cecília, Damasceno, Roberta Pacheco, Cassimiro, José & Rodrigues, Miguel Trefaut, 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3702.5.5 |
|
publication LSID |
lsid:zoobank.org:pub:E7D7BEC6-5DD6-47D6-BE26-904A69403C76 |
|
DOI |
https://doi.org/10.5281/zenodo.5696822 |
|
persistent identifier |
https://treatment.plazi.org/id/03CA87BF-B634-2E07-DFBC-B2FED880FD0A |
|
treatment provided by |
Plazi |
|
scientific name |
Crossodactylodes septentrionalis |
| status |
sp. nov. |
Crossodactylodes septentrionalis sp. nov.
( Fig. 1–3 View FIGURE 1 View FIGURE 2 )
Crossodactylodes sp. 1 – Fouquet et al. (2013: 452, 454)
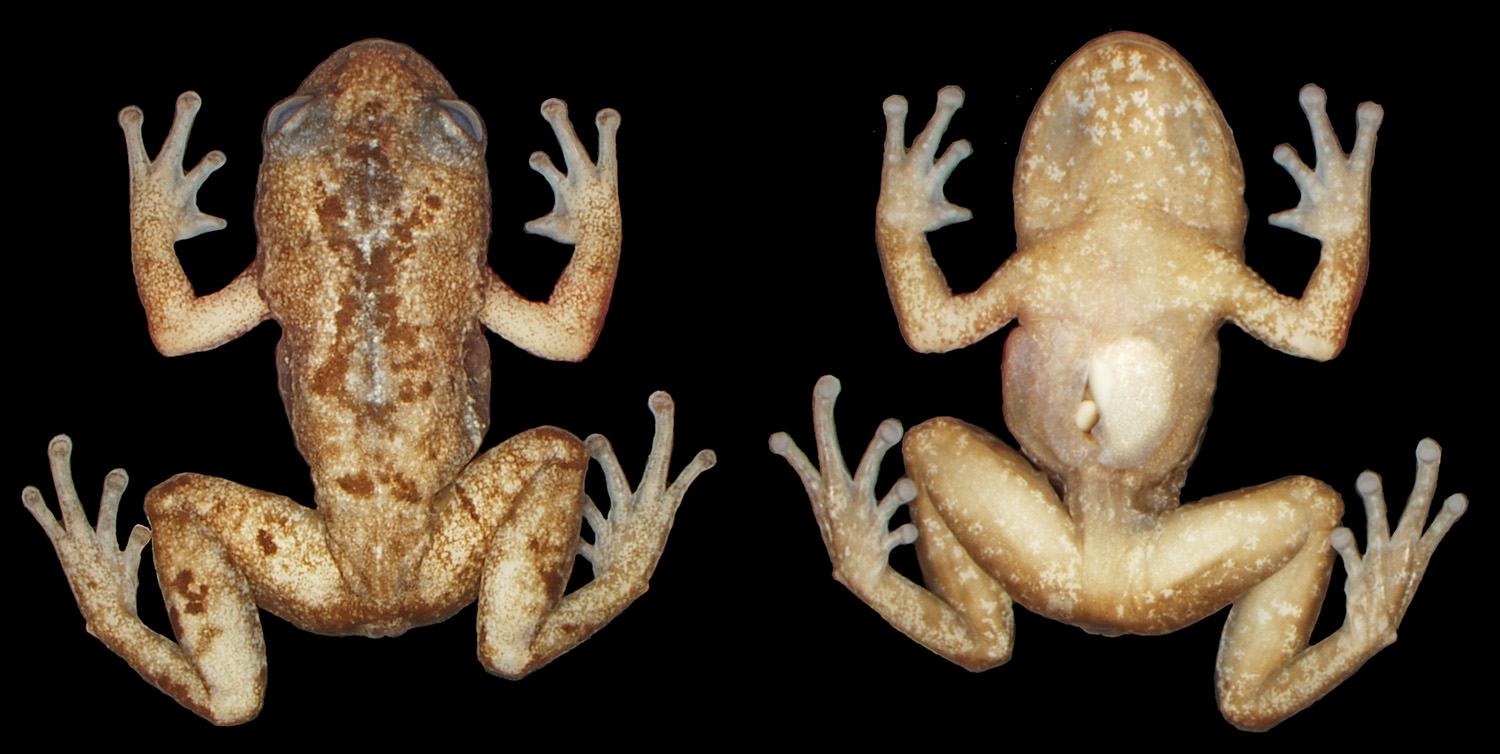
Holotype: MZUSP 150209 ( Fig. 1–3 View FIGURE 1 View FIGURE 2 ) an adult female from the summit of Peito de Moça (15°9'47.68"S, 39°20'34.03"W, 931m a.s.l.), Serra das Lontras mountain range, Arataca municipality, Bahia state, Brazil, collected by the authors on March 6th, 2009. Field number MTR 16370.
Etymology: The specific epithet septentrionalis is a Latin word that means “of the north” in reference to the geographical position where the new species was found, as it is the northernmost known Crossodactylodes species.
Diagnosis: A Crossodactylodes by the combination of columella absent, bromelicolous habits, and the close genetic similarity with the former recognized species of this genus. It is diagnosed by (1) large female body size (SVL of 17 mm); (2) snout rounded in dorsal view; (3) granules on upper eyelids absent; (4) hindlimbs without transverse bars; (5) dorsal skin granular; (6) Finger I ending in an acute tip; (7) disks on Finger III and IV rounded; (8) disks on Toes IV and V.
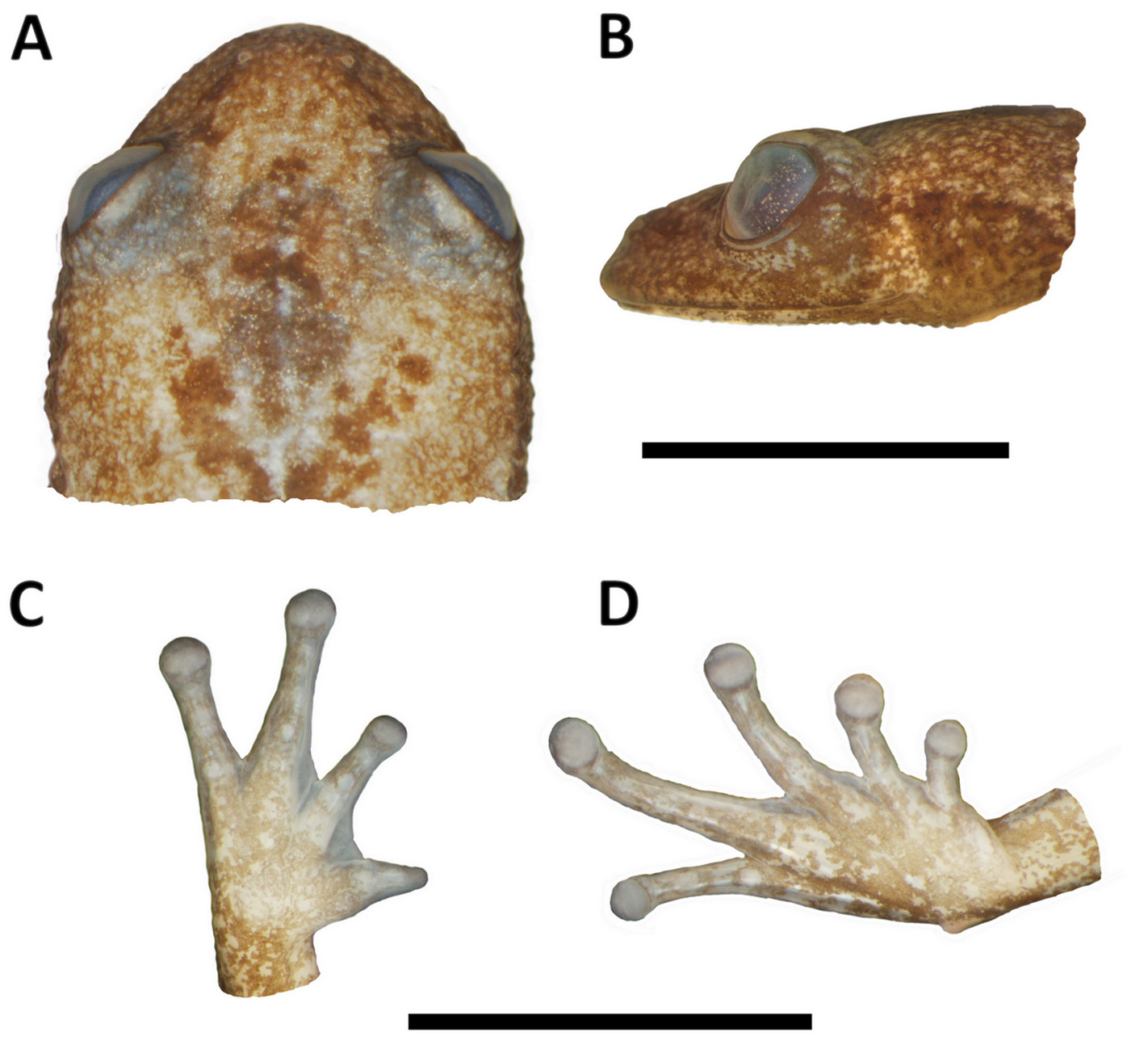
Description of the holotype: Body small, 17 mm in SVL. Head almost as long as wide (HW = 97% of HL).
Snout rounded in dorsal view, obtuse to slightly oblique in profile. Upper eyelids slightly granular. Canthal ridge blunt, loreal region slightly concave. No cranial crests. No postrictal tubercles. Eyes small, eye diameter 27% of head length, and 1.2 times the eye to nostril distance. Tympanum hidden. Nares slightly prominent, nostrils elliptical. Tongue rounded, free posteriorly. Vomerine teeth present, two on the left side, in a short row posteromedial to choanae. Choanae large, rounded, separated by a distance of about 3.5 times its diameter. Arms long and slender. Finger length I<II<IV<III; Fingers II, III, IV ending in rounded, slightly expanded disks, finger I ending in an acute tip, with no apparent disk. Fingers basally webbed; web formula I 2 - 2 - II 2 - - 3- III 3 ½ - 2 IV. Soles smooth, tubercles almost imperceptible. Carpal tubercles slightly evident. Inner carpal tubercle roughly elliptical. Outer carpal tubercle roughly rounded. Subarticular tubercles low, rounded; supernumerary slightly visible. No fringes on limbs. Legs relatively long and slender, their length (THL+TL) 85% of SVL, foot plus tarsus 57% of SVL. Toe lengths I<II <III = V<IV. Toe tips with rounded disk, slightly expanded. Toes vestigially webbed. Tarsal tubercles not evident. Inner metatarsal tubercle not prominent, large, semicircular. Outer metatarsal tubercle small, prominent. Subarticular tubercles low, rounded; supernumerary present, slightly visible (probably as an artifact of fixation process, theses tubercles are flat, and almost imperceptible). Vertebral and paravertebral regions slightly rugose, dorsolateral skin granular, ventral skin smooth, anal region smooth, anal flap slightly developed.
Color in life: Background color orange/brown tones; an irregular cream lateral line, extending from posterior edge of orbit to inguinal region, separates dorsal from ventrolateral color which is of a darker brown; a brown “X” mark on dorsum between scapulae, outlined by a whitish irregular line; whitish irregular spots on vertebral line from the middle of head to urostyle; irregular darker brown marks scattered on dorsum; dorsal surface of fore limbs orangish; ventral surface pale brown with brighten cream small irregular marks on gular region, chest, fore and hind limbs; Iris reddish.
Color in preservative: The same pattern described above, but faded. Iris becomes brown.
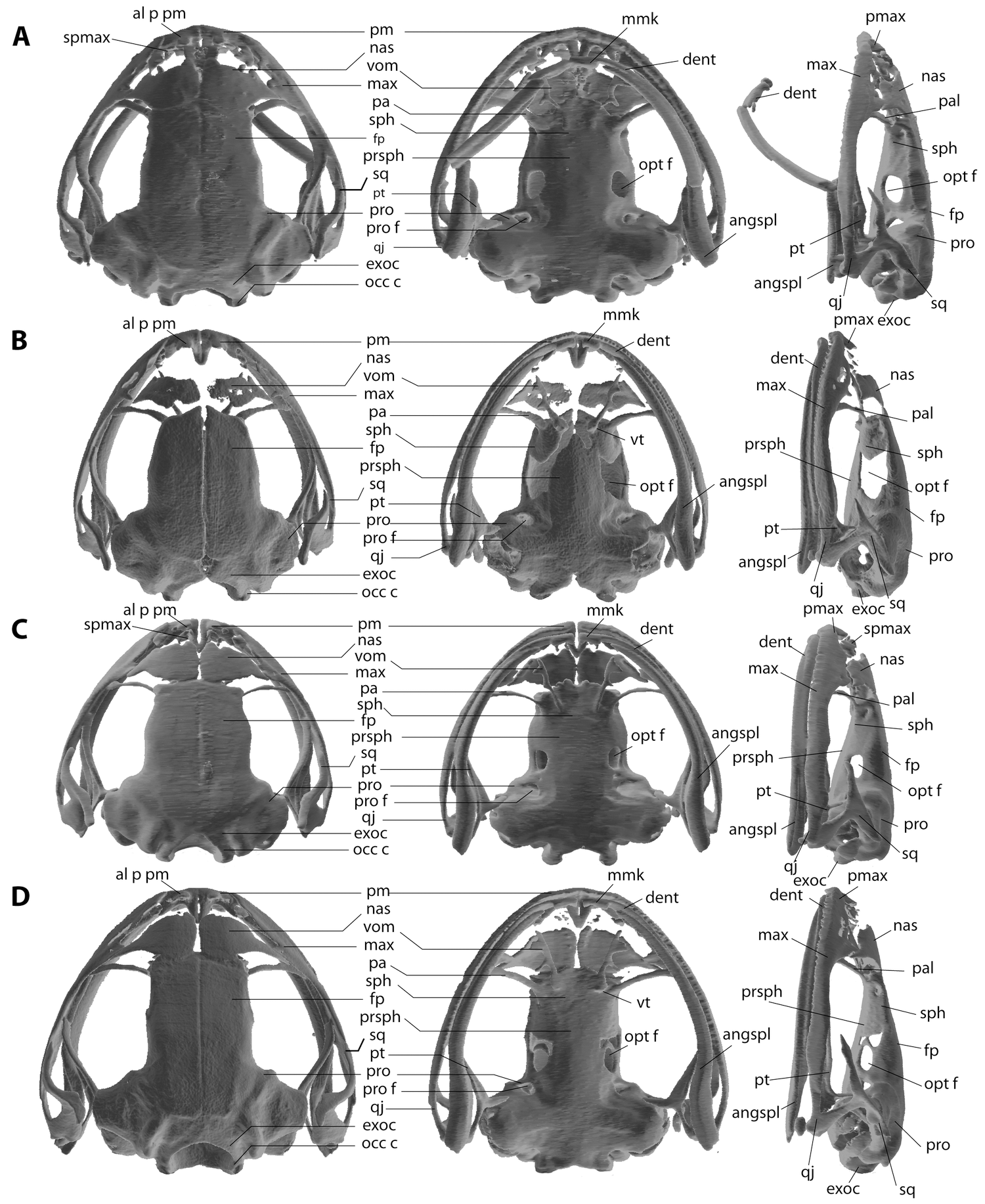
Comparison to other species (characters of the species in comparison are presented in parentheses): The new species can be distinguished from all other Crossodactylodes by having Finger I ending in an acute tip (disc of Finger I rounded), a larger body size, 17 mm SVL in the only adult female known ( C. bokermanni females 12–15 mm SVL; C. izecksohni females 11–14.4 mm SVL) and iris in vivid red (brown), and zygomatic process of squamosal bone long, passing the anterior margin of the optic foramen (short, not reaching the anterior margin of optic foramen) ( Fig. 4 View FIGURE 4 ). Additionally, the new species can be distinguished from C. bokermanni and C. izecksohni by the absence of granules on upper eyelids and by the absence of well-defined bars pattern on hindlimb (granules present; bars present). From C. bokermanni in having dorsal skin granular (smooth), rounded digital disks on Finger III and IV (elliptical), rounded digital disks on Toes IV and V (elliptical) and ventral surface of body and limbs pale brown (dark brown). By a relatively slender body when compared with C. izecksohni and C. pintoi (relatively more robust). From C. pintoi by its digital disks rounded (elliptical); rounded snout in dorsal view (truncated). From C. izecksohni and C. pintoi it can be distinguished by the presence of vomerine teeth (absent). Comparative measurements for the genus are presented in Table 1.
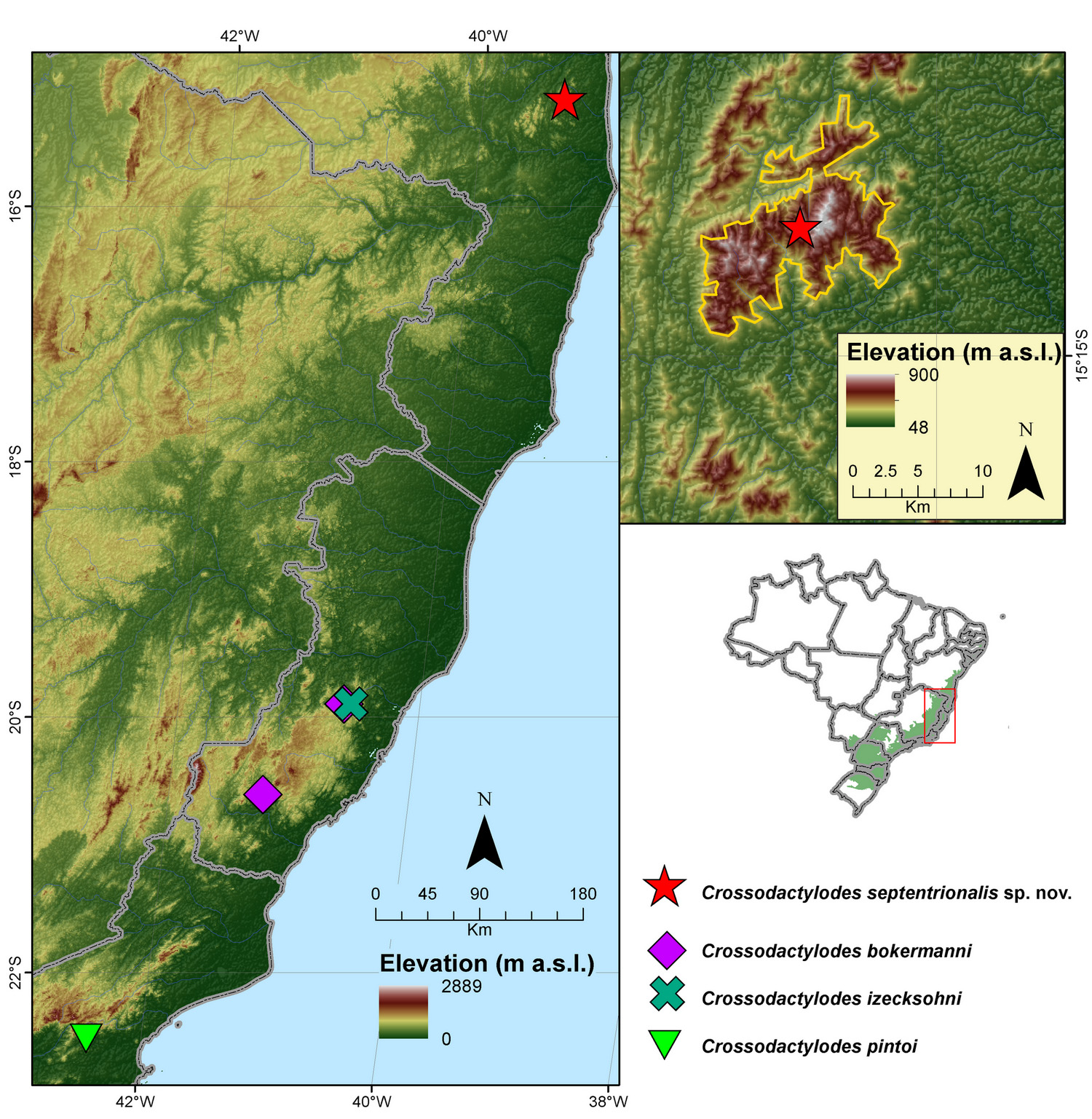
Distribution and natural history: Crossodactylodes septentrionalis sp. nov. is currently known only from the Peito de Moça peak, at Serra das Lontras, a pre-Cambrian mountain range ( Nacif et al. 2009), emerging from the low coastal plain, Arataca municipality ( Fig. 5 View FIGURE 5 ). Despite the wide altitudinal range covered by our pitfall traps, the new species was only found through active search. The holotype, the only adult obtained, was caught along with a minute juvenile at the summit of the mountain, above 930 m a.s.l., inside a 1 m diameter bromeliad. Local vegetation consists of lower and sparse trees with thick leaves; the ground is covered by a dense layer of bromeliads, herbaceous plants, lichens and mosses. Epiphytic bromeliads are also very common and some areas show a bare rock with lichens ( Fig. 6 View FIGURE 6 ). This vegetation is very distinct from that of the surrounding lower areas, which harbor much higher trees, with closed canopy producing a shaded environment. We did not detect any individuals calling.
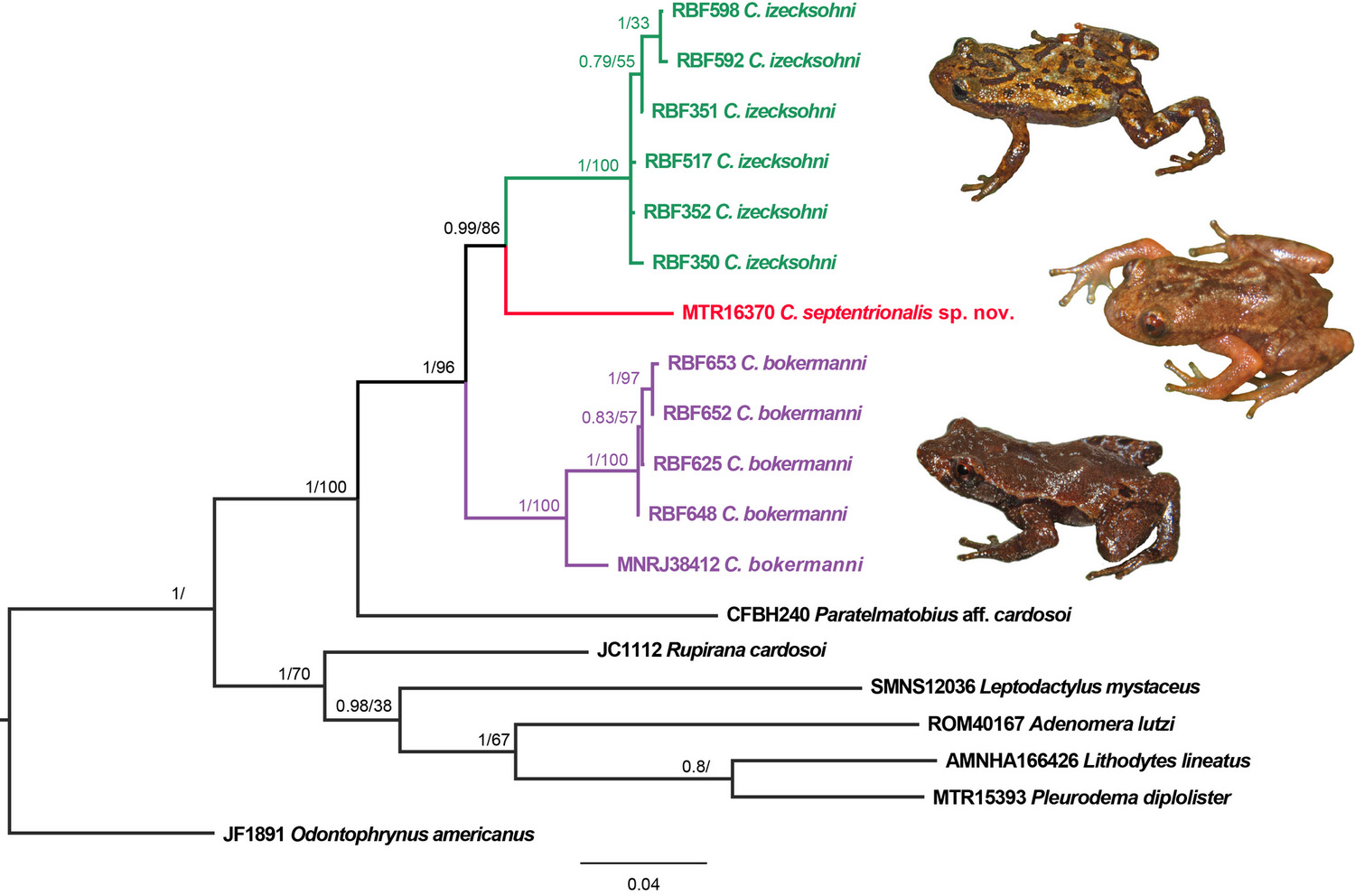
Phylogenetic relationships: All three species of Crossodactylodes included in the molecular phylogenetic analysis were recovered in a clade with high support (pp = 1; bootstrap = 96%), where the new species is sister to C. izecksohni (pp = 0.99; bootstrap = 86%) ( Fig. 7 View FIGURE 7 ). The genetic divergence between C. septentrionalis sp. nov. and C. izecksohni averaged on 5% for 16S, 6% for 12S, and ranged between 13–14% for cyt b and 5–6% for COI. Between C. septentrionalis sp. nov. and C. bokermanni differences averaged on 6–7% for 16S, 7–8% for 12S, 15% for cyt b and 5–6% for COI. Distances between C. izecksohni and C. bokermanni averaged on 5–7% for 16S, 7–8% for 12S, 14–15% for cyt b and 5–7% for COI ( Table 2 View TABLE 2 ). Genetic distances are low and fairly homogeneous between specimens of C. izecksohni from Santa Teresa. Surprisingly, this is not the case for C. bokermanni where MNRJ 38412 obtained at a close locality (Reserva Biológica de Santa Lúcia, Santa Teresa) differs strikingly from their closer relatives.
Nuclear markers are less variable than the mitochondrials (0–2% among TYR and 0–3% among RHO), but no haplotype sharing was observed for TYR among the three species of Crossodactylodes analyzed. RHO showed haplotype sharing among some individuals of C. izecksohni and C. bokermanni . Only a few heterozygous sites were found for nuclear markers among the individuals of each Crossodactylodes species (six sites for RHO, but only 3 shared by more than two individuals and three sites for TYR, and only one shared by two individuals).
Crossodactylodes was described more than seventy years ago ( Cochran 1938) and yet it is a poorly known genus endemic to the Atlantic Forests, referred only in a small fraction of papers from the growing literature on Neotropical amphibians (e.g. Cochran 1938; 1955; Lynch 1971; Peixoto 1981; 1982; Heyer 1983; Gomes 1988; Haddad & Prado 2005; Frost et al. 2006; Pertel et al. 2006; Gasparini et al. 2007; Rödder et al. 2007; Haddad et al. 2008; Langone et al. 2008; Noleto et al. 2011; Pyron & Wiens 2011; Fouquet et al. 2012).
In the original description of Crossodactylodes izecksohni and C. bokermanni, Peixoto (1982) highlights as diagnostic characters: the skin texture (smooth in C. bokermanni and granular in C. izecksohni ), body robustness (robust in C. bokermanni and slender in C. izecksohni ) and the presence of an “X” mark on dorsum (absent in C. bokermanni and present in C. izecksohni ). We have examined over 50 specimens assigned to C. izecksohni , and five of C. bokermanni , from their type locality, Santa Teresa. Although the number of examined individuals from the later species is low, they seemed fairly homogeneous in these features; however, among the specimens of C. izecksohni we found a large variation in all these characters. The dorsum varied from smooth to granular, dorsal coloration from X-marked to fairly homogenous marble-patterned and body habitus from robust to slender. This wide variation renders difficult further detection of diagnostic morphological features between C. septentrionalis sp. nov. and its congeners, especially because most diagnoses are based on males and information on females is not frequent in the literature. Nevertheless, the shape of Finger I, the digital discs, body size and molecular data can adequately separate it from all other Crossodactylodes for the moment.
Our data support previous findings for C. bokermanni and C. izecksohni showing that males reach larger SVL than females ( Gomes 1988). Accepting that the new species follows the same pattern, males of C. septentrionalis sp. nov. would have body sizes reaching up further than 17 mm of SVL, the size of the single adult female known.
Characters from internal morphology may also help intrageneric diagnoses. Gomes (1988) reported diagnostic osteological features between Crossodactylodes bokermanni and C. izecksohni (e.g. longer zygomatic process of squamosal bones in C. izecksohni , skull more elongated in profile in C. bokermanni ), which were confirmed with our osteological data. The zygomatic process of squamosal is also a diagnostic feature for C. septentrionalis sp. nov., in which it appears longer than those in both above mentioned species, and also in C. pintoi , extending beyond the anterior border of optic foramen ( Fig. 4 View FIGURE 4 ). The presence of two fenestrae just anteriorly to the optic foramen could be an additional diagnostic feature of the new species although this might be due to an incomplete closuring of sphenethmoid due to immaturity; an unlikely possibility because oviductal eggs were observed.
A suggested synapomorphy of Crossodactylodes among the Paratelmatobinae was the absence of columella ( Gomes 1988; Fouquet et al. 2013). Our data corroborates this suggestion, as it is not observed in any currently known Crossodactylodes species.
The absence of vomerine teeth was proposed to be a synapomorphy of C. izecksohni + C. pintoi ( Fouquet et al. 2013) . The new species has a small protuberance in the corresponding area of its left vomer, and a large one on the right vomer ( Fig. 4 View FIGURE 4 D). The protuberances emerge externally as a series of small vomerine teeth only at the right vomer, showing that some variation on this character may occur. In our phylogenetic hypothesis, although the monophyly of Paratelmatobinae was not recovered, probably due to the low representativeness of Leptodactylidae View in CoL in our analysis, the relationships within Crossodactylodes are well resolved, recovering C. septentrionalis sp. nov.
The molecular differences between MNRJ 38412 and other sequenced specimens of C. bokermanni living nearby at Santa Teresa, together with the morphological variation observed in C. izecksohni , also raises some questions, indicating the possibility of more undetected sympatric species. Moreover, we refrain from extending the discussion on cryptic species, as well as on character evolution in this genus, given the general limited data availability, as our osteological data comes from a single individual of each species, allowing us only to suggest hypothesis (rather than draw conclusions), and also because it will be addressed in an ongoing taxonomic revision of the genus (P. Garcia pers. comm.).
Crossodactylodes septentrionalis sp. nov. and its sister species, C. izecksohni , are currently separated from each other by more than 540 km of lowlands ( Fig. 5 View FIGURE 5 ), while C. bokermanni occurs in sympatry with C. izecksohni at Santa Teresa mountain region. This puzzling distributional pattern does not change even if the future phylogenetic analyses including C. pintoi recover it as sister to C. izecksohni . Based on the evidence available, it is difficult to suggest hypotheses on speciation scenarios that could have led to this (currently known) distributional pattern. Montane regions, however, have already been recognized as important elements driving the diversification for diverse groups ( Rodrigues et al. 2002; McCain 2005; Cadena et al. 2012; Zou et al. 2012), and this could also be the case in Crossodactylodes . The new species was only found at about 930 m a.s.l., C. bokermanni and C. izecksohni at about 650 m a.s.l. ( Peixoto 1982; Silvano & Peixoto 2004a; b), and C. pintoi, 1200 m a.s.l. ( Peixoto & Carvalho-e-Silva 2004).
These coastal mountains usually work as barriers to the moisture coming from the sea, which are pushed towards the continent by the southeasterly trade winds. This results in adiabatic cooling, leading to condensation and ultimately orographic rain ( Ackerman & Knox 2012) creating a cooler and wetter environment at higher altitudes, than those found at the surrounding lowlands. Thus, the restricted geographic distribution and the recurrent association of Crossodactylodes species with these highland habitats indicate that climate and habitat structure may have played an important role on its dispersal and isolation.
As it is currently recognized the Atlantic Forest has suffered dramatic fragmentation and expansion due climatic fluctuations of the past ( Carnaval & Moritz 2008; Carnaval et al. 2009). Thus, during these fluctuations the environmental conditions, currently found only at highland areas, may had been found also along the lowlands, allowing their ancestor to disperse; and that when the climate started to change, their optimum environment was pushed upwards, becoming restricted to the higher areas, isolating the populations and resulting in this narrow occurrence, geographically and environmentally found today. These narrow endemisms also raise concerns on the future of these species as these habitats are among those more directly threatened by climatic changes ( Williams et al. 2007). As the warmer temperatures overtake the higher elevations, the optimum microclimates for summitspecialists species would shrink or disappear, placing all Crossodactylodes species in great danger.
The new species discovered here, together with those described from this particular region in the last five years ( Cruz et al. 2008; Cruz & Napoli 2010; Recoder et al. 2010; Napoli et al. 2011; Lourenço-de-Morais et al. 2012; Teixeira Jr et al. 2012) and with other yet undescribed (pers. obs.), show that despite the exploration of the Atlantic Forest has started five centuries ago, the discoveries are still far from done. In fact they are ascending, and this severely threatened biome still harbors a highly diversified fauna waiting to be uncovered.
TABLE 2. Uncorrected p distances of mitochondrial genes among Crossodactylodes septentrionalis sp. nov., C. izecksohni, and C. bokermanni; Upper table: 16 S (below) and 12 S (above) and; Lower table: genes cyt b (below) and COI (above).
| 1 1) MTR16370 - C. | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| septentrionalis sp. nov 2) RBF350 - | 0.05 | 0.05 | 0.05 | 0.06 | 0.05 | 0.05 | 0.06 | 0.07 | 0.07 | 0.07 | 0.06 |
| C. izecksohni 0.05 3) RBF351 - | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.06 | 0.06 | 0.06 | 0.05 | |
| C. izecksohni 0.05 4) RBF352 - | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.06 | 0.06 | 0.06 | 0.05 | |
| C. izecksohni 0.05 5) RBF517 - | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.06 | 0.06 | 0.06 | 0.05 | |
| C. izecksohni 0.06 6) RBF592 - | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.07 | 0.07 | 0.07 | 0.07 | 0.06 | |
| C. izecksohni 0.05 7) RBF598 - | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.06 | 0.06 | 0.06 | 0.06 | 0.05 | |
| C. izecksohni 0.05 8) RBF625 - | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.06 | 0.06 | 0.06 | 0.06 | 0.05 | |
| C. bokermmanni 0.06 9) RBF648 - | 0.06 | 0.06 | 0.06 | 0.07 | 0.06 | 0.06 | 0.00 | 0.00 | 0.00 | 0.04 | |
| C. bokermmanni 0.07 10) RBF652 - | 0.06 | 0.06 | 0.06 | 0.07 | 0.06 | 0.06 | 0.00 | 0.00 | 0.00 | 0.04 | |
| C. bokermmanni 0.07 11) RBF653 - | 0.06 | 0.06 | 0.06 | 0.07 | 0.06 | 0.06 | 0.00 | 0.00 | 0.00 | 0.04 | |
| C. bokermmanni 0.07 12) MNRJ38412 - | 0.06 | 0.06 | 0.06 | 0.07 | 0.06 | 0.06 | 0.00 | 0.00 | 0.00 | 0.04 | |
| C. bokermmanni 0.06 1 1) MTR16370 - | 0.05 2 | 0.05 3 | 0.05 4 - | 0.06 5 | 0.05 6 | 0.05 7 | 0.04 8 | 0.04 9 | 0.04 10 | 0.04 11 | 12 |
| C. septentrionalis sp. nov 2) RBF350 - | 0.13 | 0.14 | - | 0.13 | 0.13 | 0.13 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| C. izecksohni 0.13 3) RBF351 - | 0.00 | - | 0.00 | 0.01 | 0.01 | 0.14 | 0.14 | 0.14 | 0.14 | 0.15 | |
| C. izecksohni 0.14 4) RBF352 - | 0.00 | - | 0.00 | 0.01 | 0.01 | 0.14 | 0.14 | 0.14 | 0.14 | 0.16 | |
| C. izecksohni - 5) RBF517 - | - | - | - | - | - | - | - | - | - | - | - |
| C. izecksohni 0.13 6) RBF592 - | 0.00 | 0.00 | - | 0.01 | 0.01 | 0.14 | 0.14 | 0.14 | 0.14 | 0.15 | |
| C. izecksohni 0.13 7) RBF598 - | 0.01 | 0.01 | - | 0.01 | 0.00 | 0.14 | 0.14 | 0.14 | 0.14 | 0.15 | |
| C. izecksohni 0.13 8) RBF625 - | 0.01 | 0.01 | - | 0.01 | 0.00 | 0.14 | 0.14 | 0.14 | 0.14 | 0.15 | |
| C. bokermmanni 0.15 9) RBF648 - | 0.14 | 0.14 | - | 0.14 | 0.14 | 0.14 | 0.00 | 0.00 | 0.00 | 0.07 | |
| C. bokermmanni 0.15 10) RBF652 - | 0.14 | 0.14 | - | 0.14 | 0.14 | 0.14 | 0.00 | 0.00 | 0.00 | 0.07 | |
| C. bokermmanni 0.15 11) RBF653 - | 0.14 | 0.14 | - | 0.14 | 0.14 | 0.14 | 0.00 | 0.00 | 0.00 | 0.07 | |
| C. bokermmanni 0.15 12) MNRJ38412 - | 0.14 | 0.14 | - | 0.14 | 0.14 | 0.14 | 0.00 | 0.00 | 0.00 | 0.06 | |
| C. bokermmanni 0.15 | 0.15 | 0.16 | 0.15 | 0.15 | 0.15 | 0.07 | 0.07 | 0.07 | 0.06 |
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Paratelmatobiinae |
|
Genus |
Crossodactylodes septentrionalis
| Jr, Mauro Teixeira, Recoder, Renato Sousa, Amaro, Renata Cecília, Damasceno, Roberta Pacheco, Cassimiro, José & Rodrigues, Miguel Trefaut 2013 |
Crossodactylodes
| Fouquet 2013: 452 |