Crepidacantha fasciata, Almeida & Larré & Vieira, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5048.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:3348A3F1-92B9-46D0-B567-C5BBEE68088F |
|
DOI |
https://doi.org/10.5281/zenodo.5556718 |
|
persistent identifier |
https://treatment.plazi.org/id/4B0AF9A5-EDE2-48A5-BF38-FE9CC91874A4 |
|
taxon LSID |
lsid:zoobank.org:act:4B0AF9A5-EDE2-48A5-BF38-FE9CC91874A4 |
|
treatment provided by |
Plazi |
|
scientific name |
Crepidacantha fasciata |
| status |
sp. nov. |
Crepidacantha fasciata n. sp.
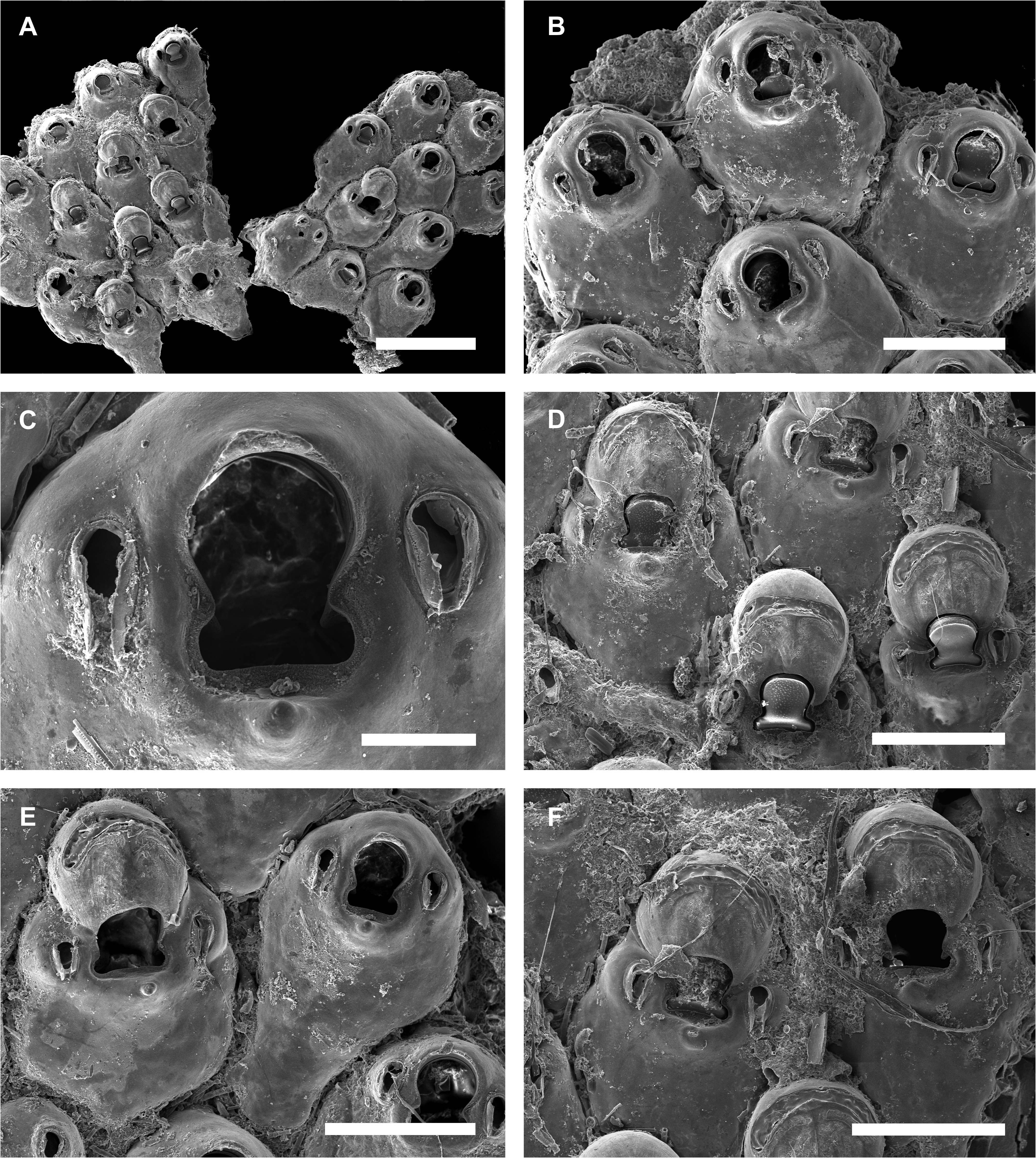
( Fig. 9A–F View FIGURE 9 )
urn:lsid:zoobank.org:act:4B0AF9A5-EDE2-48A5-BF38-FE9CC91874A4
Crepidacantha setigera: Almeida et al. 2015a: p. 5 View in CoL .
Crepidacantha longiseta: Martha et al. 2020 View in CoL : fig. 11.41c–d.
Material examined. Holotype: UFBA 690.1 , 12º51’ S, 38º10’ W, Camaçari, Costa dos Coqueiros, Bahia, Brazil, 37 m, coll. by LAMEB-UFBA, 2002 GoogleMaps . Paratypes: UFBA 1304.1 , 12º46’ S, 38º07’ W, Camaçari, Costa dos Coqueiros, Bahia, Brazil, 21 m, coll. by LAMEB-UFBA, 2008 GoogleMaps ; UFPE 889 , 4 º49’– 5º10’ S, 36º10’– 36º50’ W, Bacia Potiguar, Rio Grande do Norte, Brazil, coll. by Petrobras, 2009–2010 GoogleMaps . Additional specimens: UFBA 1305.1 , 12º47’ S, 38º07’ W GoogleMaps , Camaçari, Costa dos Coqueiros, Bahia, Brazil, 26 m, coll. by LAMEB-UFBA, 2002 ; UFBA 2888.7 , UFBA 2895 , UFBA 3631 , 13°07’ S, 38°38’ W GoogleMaps , 13–21 m, Baía de Todos os Santos , Bahia, Brazil, coll. by Ricardo Miranda, 2017 ; UFBA 1339.2 , 13°53’ S, 39°59’ W GoogleMaps , Camamu, Costa do Dendê , Bahia, Brazil, 18 m, coll. by LAMEB-UFBA, 2004 .
Diagnosis. Crepidacantha with subpentagonal autozooids with 3–4 marginal pores, 10–12 long marginal spines placed along the distolateral margins, primary orifice with straight (in autozooids) to convex (in ovicelled zoids) proximal margin, secondary orifice forming a suboral umbo, vertically arranged mediolatero-oral avicularia with short subtriangular rostrum directed proximally, and ooecium with frontal calcified ectooecium excepted by membranous distal arch which covers the porous endooecium.
Type locality. Camaçari , Bahia, Brazil .
Etymology. Latin fasciatus, banded, alluding to the distinct membranous ectooecial band.
Description. Colony encrusting, fragile, thinly calcified, unilaminar ( Fig. 9A View FIGURE 9 ).
Autozooids subpentagonal, globular (0.397–0.459– 0.584 mm long, n = 11, SD = 0.053 mm; 0.292–0.337– 0.374 mm wide, n = 11, SD = 0.024 mm), arranged quincuncially, separated by deep grooves; 10–12 long marginal spines placed along the distolateral margins, basal pore-chambers easily seen in marginal zooids ( Fig. 9B View FIGURE 9 ). Frontal shield smooth, imperforate apart from 3–4, tiny, areolar-septular pores around proximal margin. Primary orifice cleithridiate (0.092–0.098– 0.108 mm long, n = 13, SD = 0.004 mm; 0.069–0.076– 0.085 mm wide, n = 13, SD = 0.005 mm), distal margin semicircular, proximolateral constriction with a pair of triangular condyles directed downward and straight proximal margin ( Fig. 9C View FIGURE 9 ); a thick margin of calcification around the orifice with a pointed suboral umbo ( Fig. 9C, D View FIGURE 9 ).
Small, paired, latero-oral avicularia (0.053–0.061– 0.072 mm long, n = 30, SD = 0.005 mm; 0.026–0.033– 0.038 mm wide, n = 30, SD = 0.003 mm), elevated in relation to the frontal shield, directed proximally, drop-shaped, with proximal margin semicircular, short subtriangular rostrum, open-tipped, without crossbar and with long setiform mandibles ( Fig. 9C, E View FIGURE 9 ).
Ovicelled zooids have orifice with convex proximo-frontal margin; cleithral, closed by zooidal operculum, its distal half bordered by proximal corners of ooecium ( Fig. 9D View FIGURE 9 ). Ovicell hyperstomial, globular (0.165–0.183– 0.209 mm long, n = 9, SD = 0.011 mm; 0.180–0.191– 0.203 mm wide, n = 9, SD = 0.007 mm); ooecium with calcified ectooecium except for the transverse distal arch ( Fig. 9F View FIGURE 9 ).
Remarks. Some specimens from Bahia here assigned to C. fasciata n. sp. were previously misidentified as Crepidacantha setigera ( Smitt, 1873) (acc. Almeida et al. 2015a) and C. longiseta (acc. Martha et al. 2020). Crepidacantha setigera have been reported from different localities, but it is considered badly characterized since there is no recent morphological characterization of the holotype. Additionally, there are misinterpretations in the specimens assigned to C. setigera and C. longiseta from Florida and the Caribbean (e.g., Winston 1982; Winston 2016; Winston & Jackson 2021), sometimes characterized by having ectooecium with rounded membranous area or also with membranous distal arch. The holotype of Crepidacantha longiseta is deposited at the Department of Paleobiology, Smithsonian National Museum of Natural History (http://n 2t.net/ark:/65665/m3163beaa4-30d5- 4271-9c37-ca07870e40ea), and resembles part of specimens figured by Winston & Jackson (2021, fig. 118E), with frontal widely rounded membranous ectooecial area. Other specimens from Jamaican assigned to C. longiseta (e.g., Winston & Jackson 2021, fig. 188C, F) have quite distinct ovicells (and also different size of orifices and avicularia) and they belong to a distinct species. Unfortunately, description provided by Smitt (1873) is based on an imperfect specimen with no proper morphological characterization. Smitt’s description and illustration ( Smitt 1873, fig. 206) resemble those specimens reported from the Oculina reefs in Florida ( Winston 2016), characterized by ooecia with small imperforate hoods, central area of exposed endooecium and small pores. Thus, these discrepancies in species descriptions and characterization of C. longiseta and C. setigera indicate that these taxa still need a proper review. Based on the recent characterization of C. setigera (acc. Winston 2016), it differs from C. fasciata n. sp. in having ooecium with a porous fenestra and concave proximal orificial margin; in C. fasciata n. sp. the proximal orificial margin is straight or very weakly convex. Specimens previously reported from Bahia, Brazil as Crepidacantha teres ( Hincks, 1880) had oral avicularia placed horizontally below the orifice, and it is here referred to a new species (see below).
Among the living species of the genus, C. fasciata n. sp. is more similar to Crepidacantha anakenensis Moyano, 1973 , from Isla de Pascua, Crepidacantha bracebridgei Brown, 1954 , from Australia, and Crepidacantha longiseta Canu & Bassler, 1928a , from Gulf of Mexico but also reported from Brazil ( Brown 1954), in having mediolaterooral avicularia. The three species differ from C. fasciata n. sp., however, in having ectooecium with widely rounded, frontal, membranous area ( Brown 1954; Canu & Bassler 1928a; Moyano 1973); in C. fasciata n. sp. there is a distal membranous ectooecial arch, like that described in Crepidacantha carsioseta Winston & Heimberg, 1986 from Indonesia. Specimen assigned to C. longiseta from Brazil (acc. Brown 1954) requires review, which is beyond the scope of the present work.
Distribution. Western Atlantic: Brazil (Bahia and Rio Grande do Norte). Crepidacantha fasciata n. sp. can be found encrusting corals, algae and artificial substrata (PVC plates); 13‒ 37 m.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Flustrina |
|
SuperFamily |
Mamilloporoidea |
|
Family |
|
|
Genus |
Crepidacantha fasciata
| Almeida, Ana C. S., Larré, Igor R. N. M. & Vieira, Leandro M. 2021 |
Crepidacantha setigera:
| Almeida, A. C. S. & Alves, O. & Peso-Aguiar, M. & Dominguez, J. & Souza, F. 2015: 5 |