Cochranella guayasamini, Twomey, Evan, Delia, Jesse & Castroviejo-Fisher, Santiago, 2014
|
publication ID |
https://doi.org/10.11646/zootaxa.3851.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:9840D64B-F08C-44E7-B2DC-4818F8FFDD4F |
|
DOI |
https://doi.org/10.5281/zenodo.6136427 |
|
persistent identifier |
https://treatment.plazi.org/id/664887B1-FFC4-FF99-FF7C-F8F1D258FE38 |
|
treatment provided by |
Plazi |
|
scientific name |
Cochranella guayasamini |
| status |
sp. nov. |
Cochranella guayasamini View in CoL new species
Figures 19–21 View FIGURE 19 View FIGURE 20 View FIGURE 21. a, b
Cochranella croceopodes Catenazzi and Venegas 2012 View in CoL
Cochranella View in CoL sp. 2 Castroviejo-Fisher, Guayasamin, Gonzalez-Voyer, and Vilà 2014 Holotype. MHNC 13930, adult male in good state of preservation (right hind limb was cut below the knee and preserved for molecular analyses) collected from a stream near the village of San Jose, approximately 33 km (by road) northeast of Tarapoto, along the Yurimaguas road, San Martín, Peru ( 6°25'14.67"S, 76°17'28.47"W, 517 m), collected by S. Castroviejo-Fisher on March 31, 2010.
Paratopotypes. MNCN 45950 and MHNC 13929, same data as holotype.
Paratypes. MNCN 45951 and MHNC 13931, both adult males collected from a stream near the village of Santa Rosa, approximately 26 km (by road) northeast of Tarapoto along the Yurimaguas road, San Martín, Peru ( 6°24'44.79"S, 76°19'2.09"W, 686 m), collected by ET, M. Pepper, and M. Sanchez-Rodriguez on February 9, 2010; CORBIDI 8956, an adult male collected from a stream behind the village of Lejia near Abra Tangarana View in CoL on the Tarapoto-Moyobamba road, San Martín, Peru ( 6°18'46.68"S, 76°43'34.23"W, 1120 m), collected by JD and ET on June 22, 2011.
Referred specimens. CORBIDI-HE-2010-6816, collected from a stream near “Puesto de Control 15”, near the town of Shamboyacu, San Martín, Peru ( 6°56'27.18"S, 76°3'49.75"W, 955 m), collected by P. Venegas on 2 June 2010; CORBIDI-HE-2012-11392, collected from a stream in the northern Cordillera de Kampanquis, Amazonas, Peru ( 4°2'35.1''S, 77°32'28.3''W, 325 m), collected by A. Catenazzi on 12 August 2011. We identified these specimens on the basis of photographs taken by the collectors.
Generic placement. The new species is placed in the genus Cochranella on the basis of our phylogenetic analyses that included complete or partial sequences of the mitochondrial genes 12S and 16S. Additionally, the following phenotypic characters typical of Cochranella ( Guayasamin et al. 2009) are present in the new species: lobed liver covered by a transparent hepatic peritoneum; ventral parietal peritoneum white anteriorly and transparent posteriorly; moderate to extensive webbing between fingers III and IV; bones green in life; dentigerous process of the vomer and vomerine teeth present; males call from the upper surfaces of leaves, and females deposit eggs on the upper sides of leaves along streams. However, the value of these characters as synapomorphies for Cochranella needs to be tested.
Diagnosis. The following unique combination of characters distinguishes Cochranella guayasamini from other species of centrolenids: (1) a dentigerous process on each vomer (in some specimens low and hardly visible), each bearing a row of two small vomerine teeth; (2) snout rounded in dorsal view, rounded and slightly inclined in profile; (3) tympanum visible, not covered by skin, angled laterally with slight dorsal inclination, tympanic annulus low, supratympanic fold absent; (4) skin of dorsum and flanks smooth, skin of venter and thighs coarsely areolate, skin of other ventral surfaces smooth; (5) cloacal ornamentation formed by folded skin bearing small and nonenameled warts and two paired round tubercles below vent; (6) parietal peritoneum anterior half white (both on the ventral and dorsal sides), posterior half transparent, pericardium white, hepatic peritoneum transparent, visceral (stomach and intestines), renal, and gonadal peritonea mostly transparent but with some small and isolated traces of iridophores; (7) liver with three lobules; (8) adult males with a small humeral spine; (9) webbing absent between fingers I and II, basal between fingers II and III, and III 2 - 1 + IV; (10) webbing formula of toes I 1 - 2 - II 1 - (2–2-) III 1 - 2 IV (2–2-) - 1 V; (11) folds on limbs absent, fringe on postaxial edge of finger IV and toe V; (12) nuptial pad Type I, not pigmented; (13) when appressed Finger I slightly longer than Finger II; (14) eye diameter 2.5 times the width of disc on Finger III; (15) coloration in life: dorsal and dorsolateral surfaces green with yellow tones and minute melanophores (absent in the upper lip), upper rim of lip white (enameled), eyelid rims bright yellow, ventral surfaces yellowish green, bones green; (16) coloration in preservative: dorsal and dorsolateral surfaces dull cream with minute melanophores and iridophores, the former are more conspicuous on the upper eyelid making it lavender, rim of eyelids conspicuously white due to iridophores, the thin layer of iridophores of the upper lip (enameled) is almost unappreciable, ventral surfaces and flanks dull cream; (17) iris in life reddish-bronze with minute black reticulation, black or grey spots, and a black ring delimiting the iris, yellow pupillary ring absent; in preservative, iris black with minute greyish spots and a black ring delimiting the iris; (18) in life hands and feet yellowish-green with fingers and toes yellowish, melanophores absent from hands and feet including all fingers and toes; (19) males call from upper surfaces of leaves, advertisement call consisting of one to two harsh buzzes lasting 0.166– 0.28 s (note length) and with 6080–6180 Hz dominant frequency; (20) eggs golden yellow with clear jelly, clutches deposited on upper surfaces of leaves overhanging streams (N = 10), clutch size 16– 38 eggs (N = 4); (21) medium size adult males, SVL 22.5–24.5 mm (N = 5); (23) tadpoles elongate, with sinistral spiracle, oral disc not emarginate, labial tooth row formula 1/3.
Comparisons. No other species of glassfrog has a yellow rim around the eyelids in life, which are enameled white in preservative, except Nymphargus chancas and some specimens of " Centrolene " balionota. Specimens of the new species can be distinguished from N. chancas by (characters of the latter in parenthesis) webbing between external fingers developed, III 2 - 1 + IV (basal and hardly developed), snout rounded in dorsal and lateral views (truncate), dorsal coloration uniformly green (with yellow dots), iris reddish-bronze (white), humeral spine in adult males (absent). Specimens of the new species can be diagnosed from “ Centrolene ” balionota by (characters of the later in parenthesis) dorsal coloration uniformly green (green with large yellow patches and black dots), iris reddish-bronze (white), skin of flanks non-ornamented (ornamented with a longitudinal clusters of enameled glands). Within Cochranella , the only species with humeral spines is Co. litorale from the Pacific lowlands of the Choco in Ecuador and Colombia. However, this species has large black spots on its dorsum both in life and in preservative (absent in Co. guayasamini ), and lacks dentigerous processes on the vomer (present in Co. guayasamini ). Outside Cochranella , the most similar species is Centrolene durrellorum , known from the east versant of the Andes in Ecuador. However, this species has melanophores on its hands and feet including finger IV and toes IV and V (melanophores absent from hands, feet, fingers, and toes in Co. guayasamini ); dorsum lavender in preservative (cream except for the lavender upper eyelids in Co. guayasamini ), iris in life golden with a yellow pupillary ring (reddish-bronze without a yellow pupillary ring in Co. guayasamini ).
Description of holotype. Adult male with SVL of 23.4 mm. Head about 1/3 wider than body; head width 31.6% of SVL; head width 1.1 times head length. Snout rounded in dorsal view, in lateral view rounded and slightly inclined; eye-nostril distance/eye diameter 0.6 and eye-nostril distance/interorbital distance 0.8. Loreal region slightly concaved, nostrils round, flush with surrounding skin, not elevated; internarial region concave anterodorsally; canthus rostralis not well defined (rounded). Eyes medium, directed anterolaterally, eyes angled 45.0° relative to midline (where anterior-facing eyes would be 90° relative to midline); eye diameter 2.6 times wider than width of disc on finger III; eye diameter 45.9% of head length and 125.9% of interorbital distance. Tympanum visible, not covered by skin, angled laterally with slight dorsal inclination, tympanic annulus low, supratympanic fold absent. A dentigerous process on each vomer present, each bearing a row of two vomerine teeth; choanae large, ovoid, separated more widely than nostrils; tongue round, white in preservative, anterior 3/4 attached to floor of mouth; vocal slits present, extending from the sides of the base of tongue to the level of the mandibular joints. Forelimbs relatively robust, with forearm flattened and roughly 2 times as wide as arm; ulnar fold absent; humeral spine in adult males present, small, evident as an elongated bump in external view. Relative length of fingers: II <I <IV <III; finger discs wide, rounded, just larger than toe discs; hands and fingers without melanophores; webbing absent between fingers I and II, basal webbing between fingers II and III, webbing between fingers III and IV with formula III 2 - 1 + IV. Prepollex concealed; subarticular tubercles round; supernumerary tubercles absent, palmar tubercle ovoid, thenar tubercle ovoid; nuptial pad present on medial side of Finger I, running from base of finger to about halfway up finger ( Type I); glands on webbing and fringes of fingers absent. Hind limbs slender, tibia length 53.8% of SVL; tarsal fold absent; discs of toes round; feet and toes without melanophores; inner metatarsal tubercle ovoid and slightly protruding; outer metatarsal tubercle not noted. Webbing formula of feet: I 1 - 2 - II 1 - (2–2-) III 1 - 2 IV (2–2-) - 1 V. In preservative, dorsal and dorsolateral surfaces dull cream with minute melanophores and iridophores, the former are more conspicuous on the upper eyelid making it lavender, rim of eyelids conspicuously white due to iridophores, the thin layer of iridophores of the upper lip (enameled) difficult to observe, but present; dorsal skin texture smooth, skin of the venter and thighs coarsely areolate while all other ventral surfaces are smooth. Cloacal opening at level of upper thighs, partially concealed by superior dermal fold, cloacal ornamentation not conspicuous, formed by folded skin bearing small and non-enameled warts and two paired round tubercles below vent.
Coloration in life based on the type series. Dorsal and dorsolateral surfaces green with yellow tones and minute melanophores ( Fig. 19 View FIGURE 19 ). The melanophores are absent from the upper lip, hands, feet, and all fingers and toes. Upper lip rim white (enameled). Rim of eyelids bright yellow. Iris reddish-bronze with minute black reticulation and black or grey spots and a black ring delimiting the iris. Yellow pupillary ring absent. Hands and feet yellowish-green with fingers and toes yellowish. Ventral surfaces yellowish green. Bones green. Parietal peritoneum anterior half white, posterior half transparent. Hepatic and visceral (stomach and intestines) peritonea transparent. Other peritonea not visible in life.
Coloration in preservative based on the type series. Dorsal and dorsolateral surfaces dull cream with minute melanophores and iridophores ( Fig. 20 View FIGURE 20 ). The melanophores are more conspicuous on the upper eyelid making it lavender and are absent from the upper lip, hands, feet, and all fingers and toes. The thin layer of iridophores of the upper lip (enameled) is almost unappreciable, most likely lost after more than two years in 70% ethanol. Rim of eyelids conspicuously white due to iridophores. Iris black with minute greyish spots and a black ring delimiting the iris. Ventral surfaces and flanks dull cream. Parietal peritoneum anterior half white (both on the ventral and dorsal sides), posterior half transparent. Pericardium white. Hepatic peritoneum transparent. Visceral (stomach and intestines), renal, and gonadal peritonea mostly transparent but with some small and isolated traces of iridophores that seem to intrude from the dorsal portion of the parietal peritoneum. Observation made on the dissected paratype MHNC 13931.
Measurements. Holotype measurements (in mm) as follows: SVL 23.4, HL 7.4, HW 8.1, TD 1.0, IND 1.3, IOD 2.7, ED 3.4, EA 44.9°, EW 1.4, END 2.2, HaL 7.3, 3DW 1.3, TL 12.4, SL 12.6, FL 10.6. Measurements of the complete paratype series are given in Table 2 View TABLE 2 , except for MNCN 45951 (measurements not taken).
Variation. This species displays remarkably little variation across its range. Two individuals from the Cordillera de Kampanquis in northern Peru (approximately 350 km NW from the type locality) exactly match the descriptions of the type specimens. The fingers and toes of this species vary from greenish-yellow to yellowishgreen.
Tadpole. The following description is based on three preserved tadpoles of Gosner (1960) stages 26, 35 and 39 in order to observe developmental changes ( Fig. 21 View FIGURE 21. a, b ). These tadpoles were from a collected egg clutch and reared in captivity. The first tadpole (stage 26) was 28.9 mm in total length; body length 9.5 mm; body width 4.6 mm; eyeeye distance 1.4 mm (30% of body width); max. tail height 2.9 mm mid-way to tail end; tail musculature height 62% of max. tail height; body color in life translucent white. The second tadpole (stage 35) had a total length of 35.5 mm; body length 11.3 mm; body width 5.3 mm; eye-eye distance 2.8 mm (52% of body width); body color in life red, especially on ventral surfaces. The third tadpole (stage 39) reached a total length of 36.5 mm; body length 11.8 mm; body width 5.6 mm; eye-eye distance 3.8 mm (68% of body width); limb length 12.1 mm; eyes directed anterodorsally; color in life grass green. General characteristics of the tadpoles are as follows: Body round, elongate (body length 2.1 times body width); eyes dorsal, becoming more lateral as development proceeds; spiracle sinistral and lateral, vent medial; tail musculature uniform white; upper and lower jaw sheaths narrow and serrated; lateral process on upper jaw medium length with distal tips slightly curled anteriorly; oral disc not emarginate; marginal papillae on ventral side complete while completely absent on dorsal side; submarginal papillae absent; labial tooth row formula 1/ 3 in all stages examined with no gaps present.
Upon hatching, tadpoles of this species are typically reddish-gold and somewhat translucent. Over the course of development, they begin to turn bright pinkish-red, presumably due to increased vascularization near the skin surfaces ( Fig. 21 View FIGURE 21. a, b ), which may be important for respiration if these tadpoles are fossorial. However, coloration of tadpoles likely depends on local environmental conditions, such as oxygen availability ( Villa & Valerio 1982). As tadpoles approach metamorphosis, they begin to turn green although heavy vascularization is still apparent near the spine and buccal region.
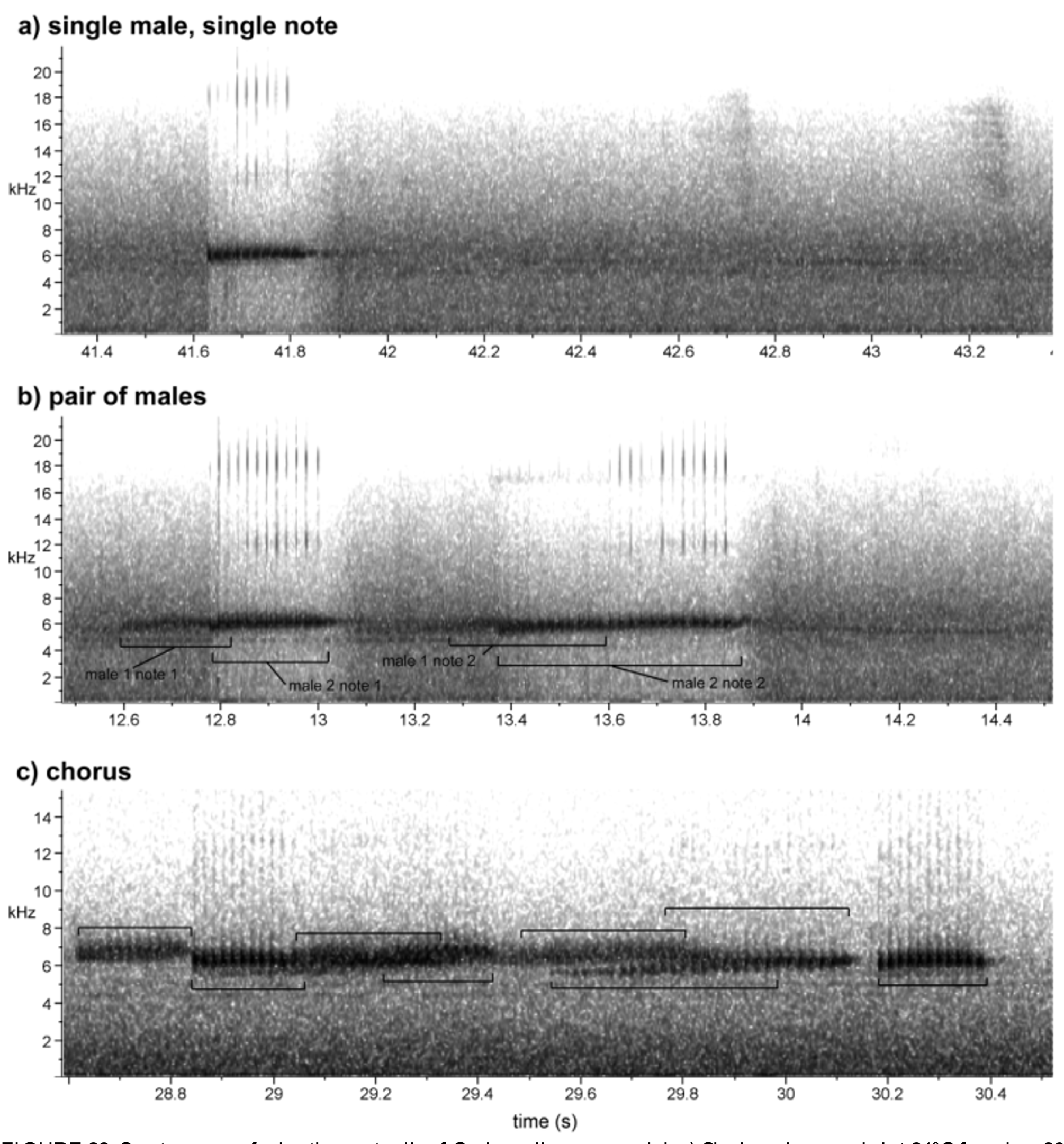
Vocalizations. The following call description comes from recordings of several different males from the Cainarachi valley at temperatures ranging from 20–24°C ( Fig. 22 View FIGURE 22 ). Two of these recordings were of two different choruses of males, one of which contained two males, the other of which contained at least four calling males. Cochranella guayasamini has a buzz-like advertisement call composed of one or two notes. The simpler one-note call consists of a single short buzz, 0.166– 0.208 s in length, composed of 9–10 pulses, pulse rate 48–54 pulses/s, dominant frequency 6080–6180 Hz. The two-note call includes a second buzz appended to the end of the first note, roughly 0.5 s after the end of the first note. This second note tends to be slightly longer in duration (roughly by 0.1 s) but is otherwise similar in structure to the first note.
One of the most striking aspects of this species’ call is its complex calling behavior. Solitary males were sometimes encountered, but in these cases males called infrequently. For example, in one stream in Abra Tangarana ( 6°16'52.87"S, 76°43'57.86"W, 1003 m), a small number of solitary Co. guayasamini were heard, each typically calling once every 10–15 min. However, in a nearby site 3.6 km S ( 6°18'46.68"S, 76°43'34.23"W, 1120 m) where Co. guayasamini were more abundant, males called in dense aggregations and each male appeared to call far more frequently, corresponding to higher frequencies of chorusing bouts (individual males typically called every 1–5 minutes). Calling behavior also becomes quite complex in group situations, as males tend to call in distinct bouts. We have one recording of a calling bout between two males, each making a two-note call ( Fig. 22 View FIGURE 22 ). The second male starts his call immediately after (and slightly overlapping with) the first note of the first male’s call, while the second notes of each male more broadly overlap. This situation becomes far more complex in larger groups (commonly with 3–6 calling males), as individual males may be making either one- or two-note calls. It seems that one male serves as the chorus leader, and his call is rapidly followed by a cascade of calls from other nearby males ( Fig. 22 View FIGURE 22 ). In the field, this gives the impression of a continuous ‘wave’ throughout the chorus, making it extremely difficult to locate individual calling males. Similar behavior in Co. granulosa was studied in detail by Ibáñez (1991), who reported that males rapidly called in response (within 816 ms) to a neighbor’s call and overlaped their calls 92–100% of the time (17–85% of all notes). Chorusing behavior in Co. guayasamini , Co. erminea , and Co. granulosa seems similar in that males call once in discrete bouts (presumably responding to neighbor’s call), then remain silent until the next calling bout. In Co. granulosa , calling bouts can become calling waves traveling along the stream up to 600 m ( Ibáñez 1991). Since high levels of call overlap can actually degrade the temporal information of the call and reduce males’ ability to attract mates in Co. granulosa ( Ibáñez 1991) , call overlap among these Cochranella may serve an anti-predator function interfering with detection ( Ryan 1986).
Distribution and ecology. Cochranella guayasamini occurs in the east-Andean versant of Peru from roughly 7° south latitude to 4° south latitude, at elevations between 250 and 1150 m ( Fig. 18 View FIGURE 18 ). In northern San Martín department (in the vicinity of Tarapoto), this species is commonly found in a wide variety of habitat types, from highly disturbed areas to primary forest. The highest density site we have found was at 1120 m near Abra Tangarana , in a stream flowing through a cattle pasture with low bushes growing along the edges. Prior to August 2011, this species was only known from northern San Martín. A record by Catenazzi and Venegas (2012) from the Cordillera de Kampanquis in northern Peru extends the known distribution of this species roughly 400 km NW from the type locality.
Near San Jose we monitored 10 clutches of Cochranella guayasamini over several nights (typically involving 2–3 checks per night), and did not observe any parental behavior. At oviposition eggs are bi-colored, light-to-dark brown. As in clutches of Co. granulosa , all 10 clutches were deposited on the upper surfaces of leaves, typically near the drip tip or near the leaf margin ( Fig. 21 View FIGURE 21. a, b ). Clutch size ranged from 16– 38 eggs (average = 29 ± 9.83, N = 4).
Cochranella guayasamini is sympatric with a number of centrolenid species. In northern San Martín, it has been found in streams containing the following species: Nymphargus chancas , Espadarana fernandoi , Rulyrana saxiscandens , Hyalinobatrachium pellucidum , H. carlesvilai , Chimerella corleone (which share the same type locality), and Teratohyla midas . In the Kampanquis site, Co. guayasamini was found in a stream also containing Chimerella mariaelenae .
Etymology. The name is a patronym for Juan Manuel Guayasamin ( Fig. 7 View FIGURE 7 ), an Ecuadorian herpetologist, and is a noun in the genitive case. We name this species after our dear friend in recognition of his contributions to glassfrog systematics, taxonomy, and natural history.
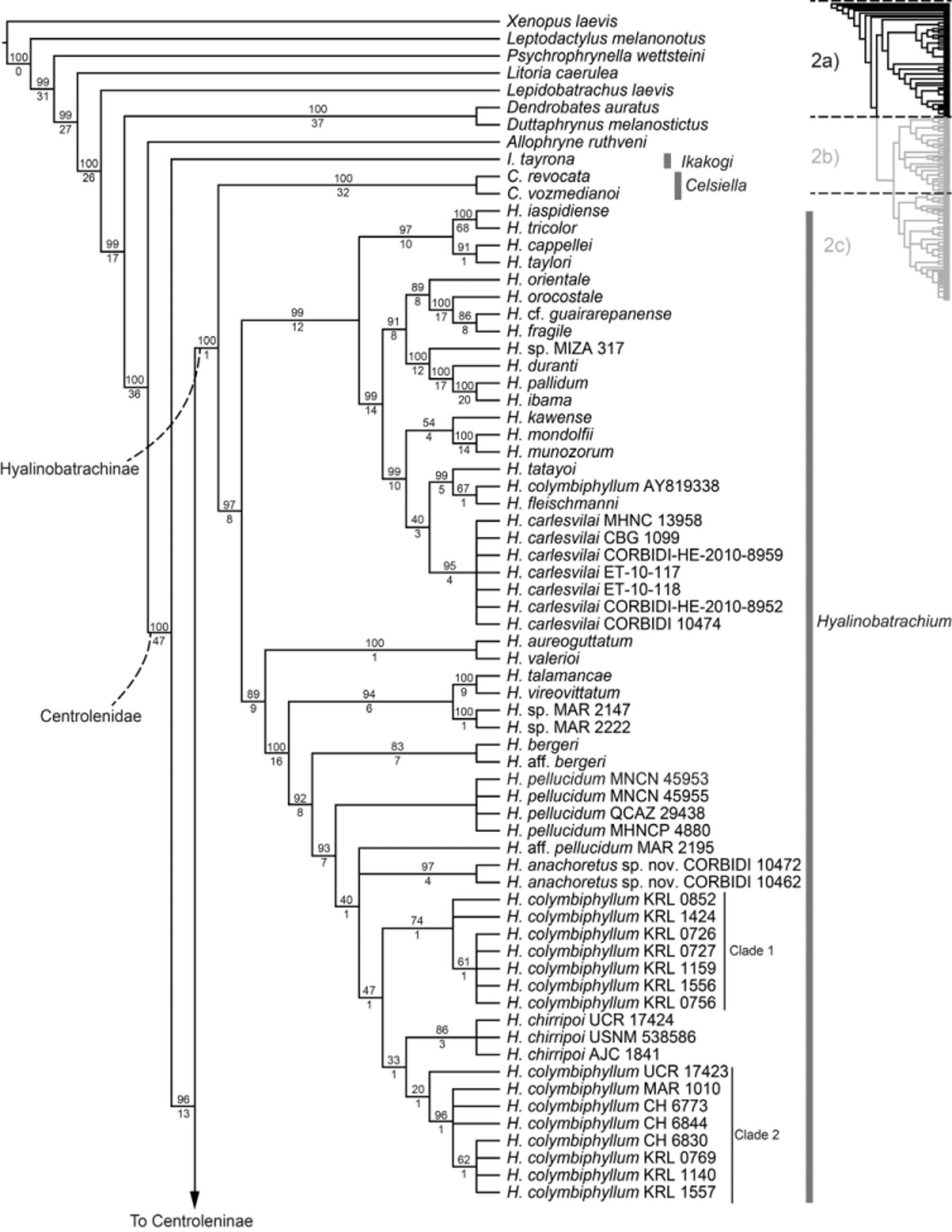
Remarks. This species has been previously misidentified as Cochranella croceopodes ( Catenazzi & Venegas 2012) . However, we provide evidence that Co. croceopodes is a synonym of Rulyrana saxiscandens (see below in the New Data section under Rulyrana saxiscandens account). Our molecular analyses recovered Co. guayasamini as a member of the genus Cochranella , and sister to Co. erminea ( Fig. 2c View FIGURE 2 a View FIGURE 2 c ). With the description of Co. guayasamini there are currently two independent origins of humeral spines in the genus Cochranella (the other in Co. litoralis ).
TABLE 2. Measurements of the type series (except MNCN 45951) of Cochranella guayasamini new species.
| MHNC 13929 | MHNC 13930 | MHNC 13931 | MNCN 45950 | CORBIDI 8956 | |
|---|---|---|---|---|---|
| SVL | 23.9 | 23.4 | 23.5 | 22.5 | 24.7 |
| HL | 7.1 | 7.4 | 7.4 | 6.5 | 7.6 |
| HW | 8.0 | 8.1 | 8.0 | 8.0 | 8.4 |
| TD | 0.8 | 1.0 | 0.9 | 0.7 | 1.0 |
| IND | – | 1.3 | 1.8 | – | 1.6 |
| IOD | – | 2.7 | 2.8 | – | 2.8 |
| ED | 3.4 | 3.4 | 3.4 | 3.2 | 3.4 |
| EA (degrees) | – | 44.9 | 38.3 | – | 40.8 |
| EW | 1.8 | 1.4 | 1.6 | 1.8 | 1.7 |
| END | – | 2.2 | 2.1 | – | 2.0 |
| HaL | – | 7.3 | 7.0 | – | 7.1 |
| 3DW | 1.3 | 1.3 | 1.4 | 1.4 | 1.3 |
| TL | 11.9 | 12.4 | 12.9 | 11.6 | 13.2 |
| SL | 11.5 | 12.6 | 12.8 | 12.2 | 12.8 |
| FL | 10.1 | 10.6 | 9.7 | 9.0 | 10.4 |
| sex | M | M | M | M | M |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cochranella guayasamini
| Twomey, Evan, Delia, Jesse & Castroviejo-Fisher, Santiago 2014 |
Cochranella croceopodes
| Catenazzi and Venegas 2012 |