Cirriformia crassicollis ( Kinberg, 1866 )
|
publication ID |
https://doi.org/10.5281/zenodo.198228 |
|
DOI |
https://doi.org/10.5281/zenodo.6209190 |
|
persistent identifier |
https://treatment.plazi.org/id/03BF2024-072A-FF87-2ADD-9B1CFC29F8B3 |
|
treatment provided by |
Plazi |
|
scientific name |
Cirriformia crassicollis ( Kinberg, 1866 ) |
| status |
|
Cirriformia crassicollis ( Kinberg, 1866) View in CoL
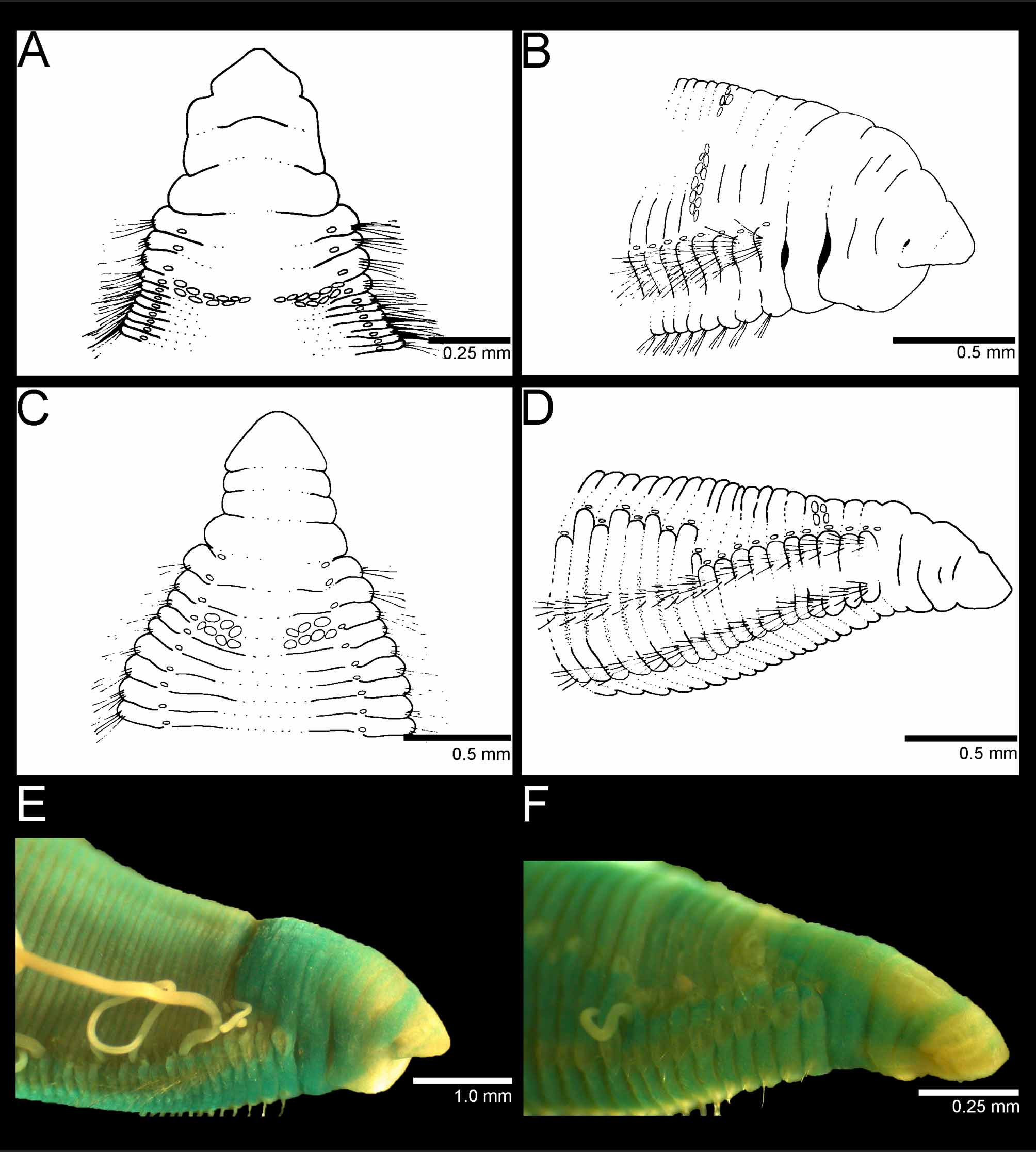
Figures 1 View FIGURE 1 (A, B, and E) and 2 (A–F)
Labranda crassicollis Kinberg, 1866 : p. 255.
Cirriformia crassicollis View in CoL ; Hartman, 1948: p. 112; Hartman, 1966: p. 226; Bailey-Brock, 1987: p. 371.
Material examined. Type material: Holotype ( SMNH 498), from Honolulu, 21°19΄ 157°52΄ (Honolulu Harbor) Leg. Eugenie Exp. 1851–53 sta. 1074-77. Non-type specimens reported by Bailey-Brock (1984) and Dreyer et al. (2005) from samples collected at Niu Valley Beach Park, south coast of Oahu Island, 21°16΄41΄ 157°44΄40΄, coll., S. A. McCarthy, W. Estabrooks; # 2 Jan 1979 (5, USNM 1145381), # 3 Jan 1979 (1, USNM 1145380), # 10 Sep 1992 (5, SMNH 110719), # 2 Feb 1977 (7), #3 1978 (11), # 3 Jan 1979 (2), #1 1979 (4), #2 (33); 11 Sep 1992 (pre Iniki hurricane) #1 (2), #3 (6), #5 (8), #7 (7), #9 (12); 5 Sep 1992 (post Iniki hurricane) #1 (3), #2 (14), #4 (2), #5 (10), #6 (4), #8 (4), #10 (5); Hanauma Bay, Keyhole, 21°16΄14΄ 157°41΄45΄, from hard substrate, 17 Aug 1999, coll. J.H. Bailey-Brock (1).
Redescription of holotype. Holotype fragmented in several regions, containing body walls and body fragments without the body wall, with all in poor condition. Hartman (1948) previously described these fragments. Prostomium had a few dark spots ( Hartman, 1948) but now, the holotype lacks the prostomium. Tentacular filaments arise above chaetiger 5 in two patches of filaments (5–7 each). Fragment 2.8 mm long, 2.0 mm wide (from left notopodium to right notopodium); six chaetigers remain on right side and ten chaetigers on left. Notopodium and neuropodium with yellow capillary chaetae number of 8–12 per fascicle. Larger piece of body wall (6.0 mm long with 4.6 mm wide) with only capillary chaetae in first chaetigers (eight chaetigers on neuropodium and 17 on notopodium only with capillaries). After that, 3 or 4 acicular spines alternate with 2–3 capillaries present. Distal part of this fragment with branchiae arising at a discrete distance from notopodial ridge (also reported by Hartman 1948).
Largest body fragment lacks body wall and about 7 mm long and 2 mm wide with more than 30 chaetigers. Fragment rounded dorsally, ventral groove. Lateral projection of parapodium forms lateral shoulder. Yellow, slightly curved acicular spines present in fragment.
Redescription. Specimens 2.5–50.0 mm long, 0.1–4.0 mm wide for 33–246 chaetigers. Body elongated, dorsally inflated, ventral surface with deep groove formed by projection of neuropodia ventrally. Smaller specimens with ventral surface flattened. First four chaetigers without distinct segmental markings, with dorsal surface resembling a dorsal crest. Crowded segments with well developed lateral shoulders. Color in alcohol yellow to tan, with branchial and tentacular filaments pale; color in life not observed.
Prostomium triangular, pointed, almost as long as wide with pair of nuchal organs located near posterolateral border ( Fig. 1 View FIGURE 1 A, B; 2A, B). Peristomium with two annuli and sub-annulations, the first annulus as large as four thoracic chaetigers and second one as large as two thoracic chaetigers ( Fig. 1 View FIGURE 1 B; 2B). Junction between peristomial annulations deeply grooved ( Fig. 1 View FIGURE 1 B; 2B).
Tentacular filaments formed by two groups of filaments (8–12) organized in two rows each, dorsally located on chaetiger 4 or 5 on most specimens. Juveniles with tentacles on chaetiger 3 ( Fig. 2 View FIGURE 2 F). Typically, two clusters positioned on only one chaetiger and this appears to be size-dependent ( Fig. 1 View FIGURE 1 A and 3B). First pair of branchiae on chaetiger 1, one branchia per parapodium, well above notopodial ridge, more numerous on anterior end of body, present in posterior chaetigers. Largest specimen with branchial filaments continuing after chaetiger 50, inserted at discrete distance from notopodial ridge. However, branchial filaments do not become mid-dorsal as in Timarete species, but remain inserted laterally.
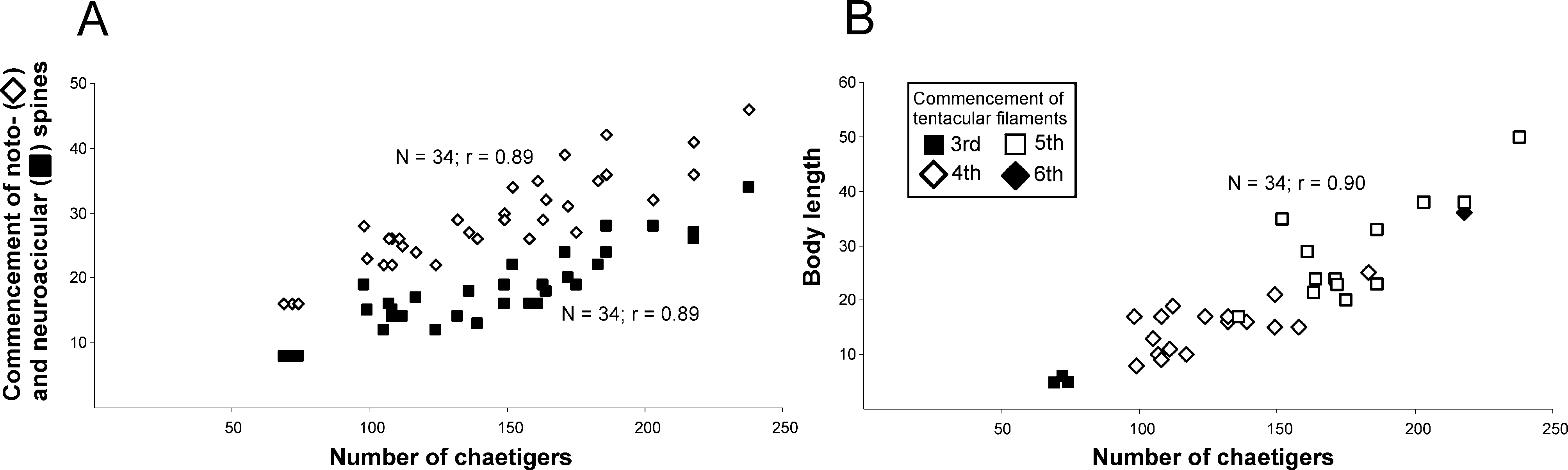
Notopodia and neuropodia widely separated. On first five chaetigers, notopodia more dorsal and neuropodia more lateral; from chaetiger 6 notopodia become more lateral and neuropodia ventral ( Fig. 2 View FIGURE 2 B). Anterior parapodia with large, yellow serrated capillary chaetae arranged in two rows ( Fig. 2 View FIGURE 2 C). Notoacicular spines first present in neuropodia in all specimens examined from chaetigers 8–34 with an average gap of approximately 10 chaetigers until their appearance in notopodia ( Fig. 3 View FIGURE 3 ). Acicular spines amber in color, slightly curved on anterior end; 3–4 spines (rarely 5) alternating with 2–3 capillary chaetae throughout ( Fig. 2 View FIGURE 2 D).
Pygidium simple ventral lip with terminal anus ( Fig. 2 View FIGURE 2 E).
Methyl green staining pattern. Prostomium with anterior tip not staining; dorsal junction with peristomium intensely stained ( Fig. 1 View FIGURE 1 E). Second peristomial annulation and anterior chaetigers before dorsal tentacles staining intensely showing dorsal crest ( Fig. 1 View FIGURE 1 E). Branchial and tentacular filaments staining weakly. Mid-segmental areas staining intensely while inter-segmental areas not staining. Notopodial and neuropodial ridges not staining while parapodia stain intensely. Ventral groove weakly stained.
Remarks. None of the current descriptions of C. crassicollis (i.e. Kinberg 1866, Hartman 1948, 1966, Bailey-Brock 1987) are sufficiently detailed to correctly identify this species. Cirriformia crassicollis differs from other congeners by the general shape of the adult body including the well developed parapodial ridge forming “shoulders”, and a deep ventral groove along the body; the tentacular filaments occur in only one chaetiger (chaetiger 4 or 5 in adult specimens), there is a gap of approximately ten chaetigers between the commencement of neuro- and notoacicular spines, and a distinct methyl green staining pattern that shows a strong reaction at the junction between the prostomium and peristomium and on the second peristomial annulation.
Juvenile individuals of this species presented only capillary chaetae throughout, less than 40 chaetigers, and tentacular filaments located on chaetiger 3 ( Fig. 2 View FIGURE 2 F). Additionally, the branchial filaments of juveniles had a few black spots sparsely distributed over their length. Blake (1996) reported different bifid spines in juveniles of Cirriformia tentaculata and C. moorei that were not present in adults.
Cirriformia crassicollis View in CoL differs from the other Hawaiian species C. semicincta (Ehlers 1905) View in CoL on the position of the tentacular filaments. In C. semicincta View in CoL the tentacular filaments arise over chaetigers 3 and 4 ( Abbott 1946, Okuda 1937) while in C. crassicollis View in CoL they arise above only one chaetiger, generally 4 or 5.
Biology. Cirriformia crassicollis were collected in Diopatra dexiognatha Paxton & Bailey-Brock, 1986 mounds; dense aggregations were found in a specific intertidal area of Honolulu. Cirriformia crassicollis (as Cirriformia sp. – Bailey-Brock 1984 and as Cirratulidae – Dreyer et al. 2005) was one of the most numerous polychaetes in these communities. Some specimens of C. crassicollis were found actually inhabiting Diopatra tubes (Bailey-Brock, pers. obs.). Bailey-Brock (1979) reported this species as a component of chaetopterid mounds on a fringing reef, south shore of Oahu, reaching densities of 200 ind./m².
Distribution. The type locality for C. crassicollis is Honolulu, Oahu, Hawaii. A new record from Halape, Big Island, Hawaii, was reported by Hartman (1966) and Bailey-Brock (1979) reported C. crassicollis from Fort Kamehameha Ahua reef near Pearl Harbor entrance channel, Oahu, Hawaii. All the specimens herein analyzed were from Niu Valley Beach Park, south coast of Oahu, and Hanauma Bay, east coast of Oahu, Hawaii. Therefore, C. crassicollis is endemic to the Hawaiian Islands.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cirriformia crassicollis ( Kinberg, 1866 )
| Magalhães, Wagner F. & Bailey-Brock, Julie H. 2010 |
C. semicincta
| Ehlers 1905 |
Labranda crassicollis
| Kinberg 1866 |