Chloeia zibrowii, Salazar-Vallejo, 2023
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5238.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:768E9932-2D18-4115-8359-3FF800328BCD |
|
DOI |
https://doi.org/10.5281/zenodo.7641443 |
|
persistent identifier |
https://treatment.plazi.org/id/03C79010-FF81-D764-FF70-7E7C268CFB2E |
|
treatment provided by |
Plazi |
|
scientific name |
Chloeia zibrowii |
| status |
sp. nov. |
Chloeia zibrowii sp. n.
urn:lsid:zoobank.org:act:98C45DCE-49BF-4F8B-83A1-9733ECC7C771
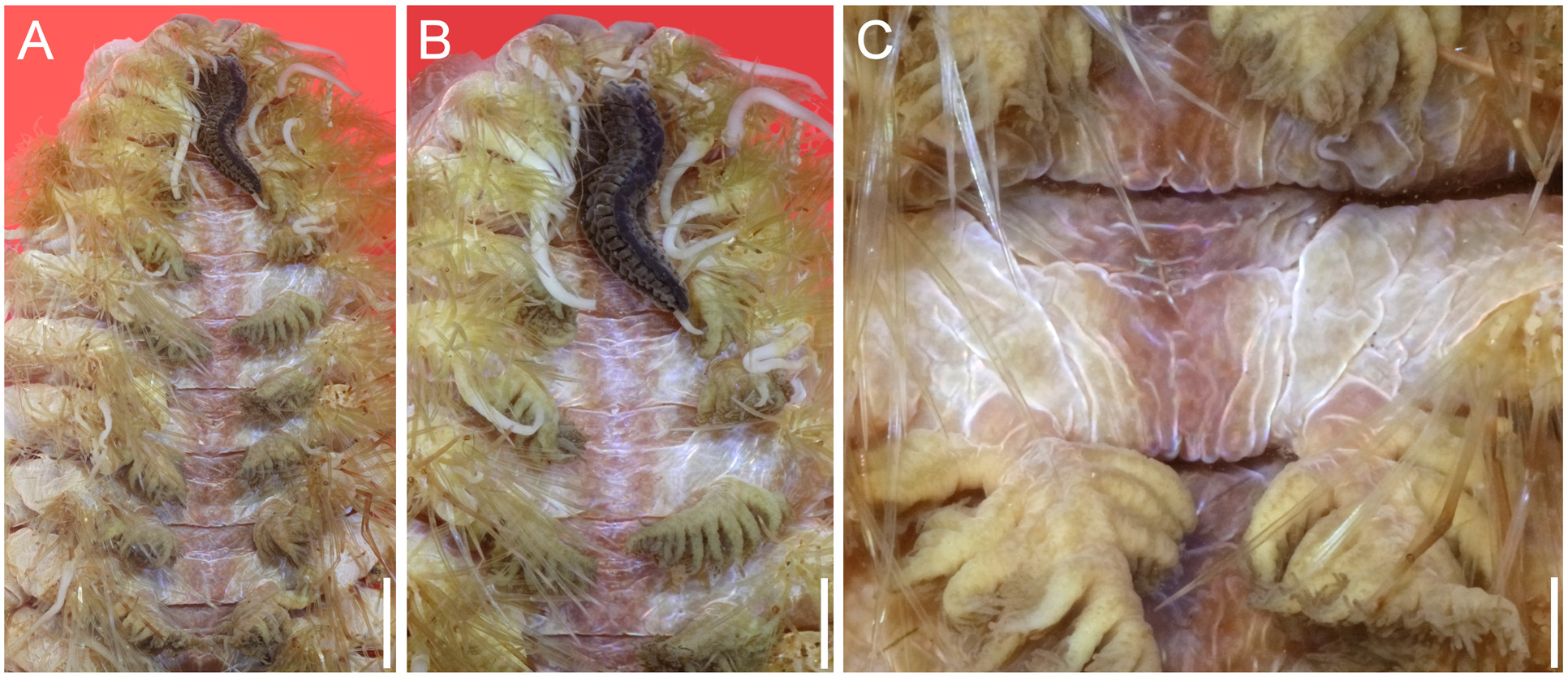
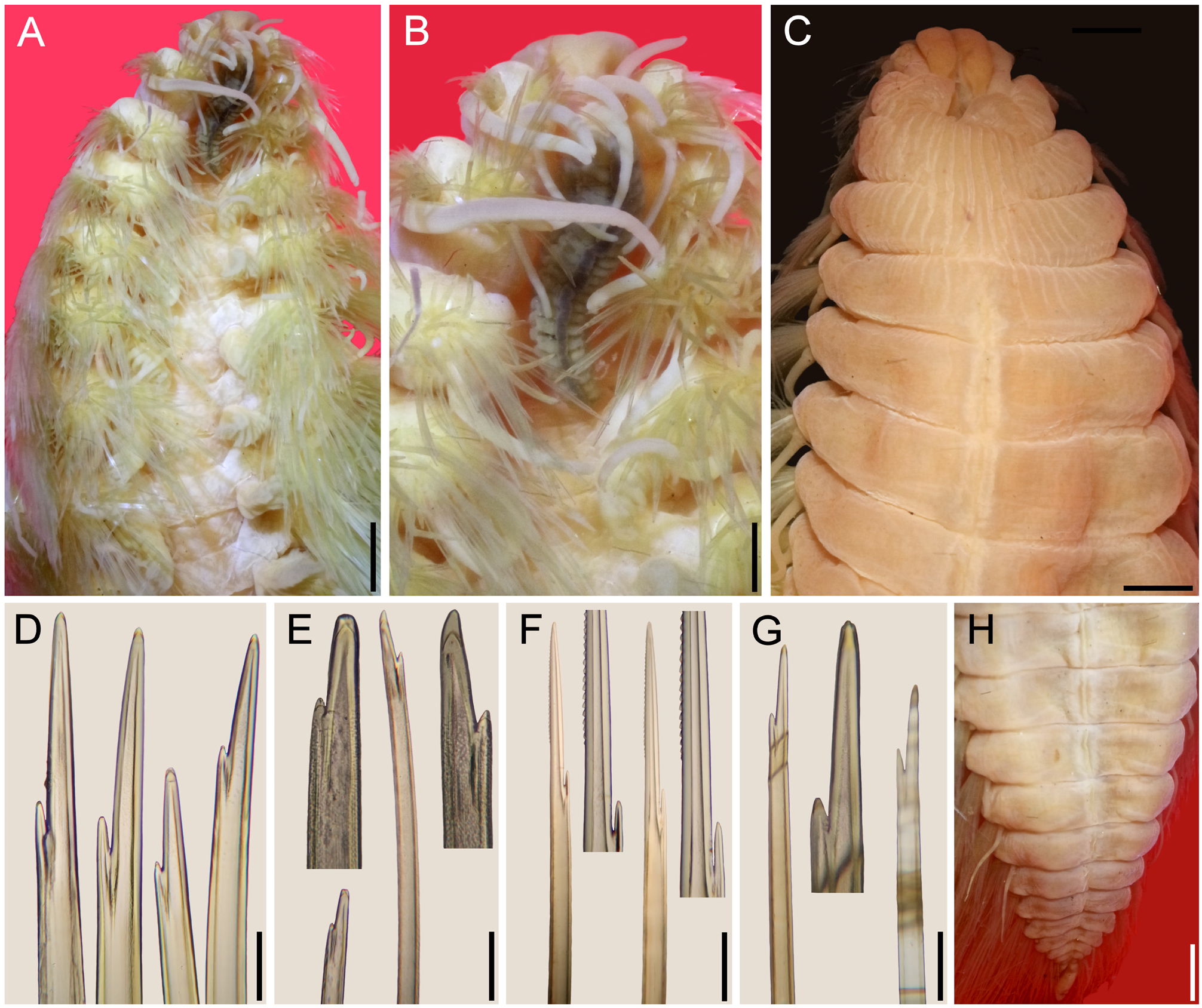
Figs 58 View FIGURE 58 , 59 View FIGURE 59
Type material. French Polynesia, Marquesas Islands.
Holotype ( MNHN IA-TYPE 2056 ), RV Alis , Sta. DR1298 (08°57.4´S, 140°01.9´W), off Nuku Hiva Island , rock dredge, 305 m, 9 Sep. 1997. GoogleMaps Five paratypes (3: MNHN IA-TYPE 2057 ; 2: ECOSUR 310), RV Alis, Sta. DR 1197 (08°57.4´S, 140°01.9´W), off Nuku Hiva Island, rock dredge, 350 m, 27 Aug. 1997 (complete MNHN paratype used for description; MNHN paratypes anterior fragments, one with most chaetae broken; 22–32 mm long, 10–13 mm wide, 12–17 chaetigers; ECOSUR paratypes one complete, markedly bent laterally, with most chaetae broken, 60 mm long, 17 mm wide, 33 chaetigers; an anterior fragment 30 mm long, 9 mm wide, 17 chaetigers; some features used for variation). GoogleMaps One paratype ( ECOSUR 311 View Materials ), RV Alis , Sta. DR1298 (08°49.1´S, 140°17.1´W), off Nuku Hiva Island, rock dredge, 305 m, 9 Sep. 1997 (without posterior end; caruncle black; eyes reddish; median antenna half as long as caruncle; cirriform branchiae with tips blackish; bipinnate branchiae with brownish lateral branches; middorsal bands almost faded; body 64 mm long, 16 mm wide, 27 chaetigers). GoogleMaps
Additional material. French Polynesia, Marquesas Islands. Two specimens (1: MNHN; 1: ECOSUR), RV Alis , Sta. CP1176 (08°44.8´S, 140°14.5´W), off Nuku Hiva Island , bean trawl, 260 m, 25 Aug. 1997 (complete; dorsum pale; median antenna 1/3 as long as caruncle, caruncle blackish; eyes blackish; cirriform branchiae with tips blackish; body 64–78 mm long, 15–18 mm wide, 33–34 chaetigers). GoogleMaps Five specimens (3 MNHN, 2 ECOSUR), RV Alis , Sta. CP1251 (09°47.2´S, 139°38.2´W), Dumont d’Urville Deep, 500–650 m, beam trawl, 2 Sep. 1997 (colorless, many chaetae broken; complete, anterior fragment 30 mm long, 11 mm wide, 15 chaetigers; smaller complete specimens with anal cirri 6–7× longer than wide, 5× in larger specimens; 22–67 mm long, 5–12 mm wide, 29–32 chaetigers) GoogleMaps .
Diagnosis. Chloeia with bipinnate branchiae from chaetiger 4, decreasing in size posteriorly, each with 11–12 lateral branches in median segments; middorsal band wide, tapered posteriorly; caruncle blackish, with about 24 folds; notochaetae harpoon shaped with spurs; neurochaetae spurred and furcates.
Description. Holotype (MNHN IA-TYPE 2056) an anterior fragment, 40 mm long, 17 mm wide, 21 chaetigers. Complete paratype (MNHN IA-TYPE 2057) complete, bent laterally; body tapered, 53 mm long, 9 mm wide, 32 chaetigers.
Holotype with an ill-defined T-shaped brownish dorsal band, with paler core in a few anterior chaetigers ( Fig. 58A View FIGURE 58 ), solid along following ones; lateral bands poorly defined, paler; caruncle black, middorsal ridge with 26 vertical folds ( Fig. 58B View FIGURE 58 ); eyes reddish, in a slightly darker area, anterior eyes 2–3× larger than posterior ones; cirriform branchiae with black tips; bipinnate branchiae brownish with darker lateral branches, each with 6–7 filaments per side, with darker tips ( Fig. 58C View FIGURE 58 ); many chaetae broken. Venter brownish, with a pale midventral band.
Complete paratype (MNHN IA-TYPE 2057) pale. Dorsum without pigmentation ( Fig. 59A View FIGURE 59 ). Dorsal cirri pale. Interramal areas pale. Bipinnate branchiae pale. Venter paler, with a whitish band ( Fig. 59C View FIGURE 59 ).
Prostomium anteriorly entire, anterior prostomial area pale ( Fig. 59B View FIGURE 59 ). Eyes reddish, anterior eyes 2–3× larger than posterior ones. Median antenna inserted at anterior caruncular margin, pale, almost half as long as caruncle (4/5 as long in one additional specimen), apparently 2× longer than laterals (without tips). Lateral antennae bases close to each other. Palps broken, slightly narrower than lateral antennae. Mouth ventral on chaetiger 2. Pharynx not exposed.
Caruncle blackish, trilobed, slightly curved, reaching chaetiger 4; middorsal ridge black, with 24 vertical folds. Lateral lobes narrow, barely visible, with about 30 vertical folds.
Bipinnate branchiae from chaetiger 4, continued throughout body, parallel along most body segments, progressively larger to chaetigers 13–16, diminishing in size in posterior chaetigers. In median segments each branchia with 11–12 lateral branches.
Parapodia biramous, notopodia with cirriform branchiae along chaetigers 1–3, progressively smaller, 4/5–1/3 as long as dorsal cirri; one complete with black tip. Dorsal cirri about 2× longer than branchiae in median and posterior chaetigers. Second ventral cirri with cirrophores 2–3×longer and wider than adjacent ones, and cirrostyles 2× longer than adjacent ones, directed dorsally. Other ventral cirri directed ventrolaterally, as long as two segments in median region, up to three segments in posterior ones.
Chaetae whitish to golden, neurochaetae with a pale green hue (observed in one paratype). Complete chaetae with distal fragile hoods. Notochaetae in anterior chaetigers spurred and furcates ( Fig. 59D View FIGURE 59 ), major tines 5–12× longer than minor ones. Median chaetigers with spurred, harpoon-chaetae ( Fig. 59F View FIGURE 59 ), denticulate tines 9—12× longer than smooth ones. Neurochaetae spurred and furcates with tiny minor tines, anterior chaetigers with major tines 5—8× longer than minor ones ( Fig. 59E View FIGURE 59 ), median chaetigers with major tines 4–10× longer than minor ones ( Fig. 59G View FIGURE 59 ).
Posterior region tapered ( Fig. 59H View FIGURE 59 ); pygidium with anus terminal; anal cirri whitish, digitate, 5–6× longer than wide.
Live pigmentation. Unknown.
Variation. Incomplete paratypes (MNHN IA-TYPE 2057) with body wall darker than complete paratype. Eyes reddish, anterior eyes 2–3× larger than posterior ones. Caruncles darker than in complete paratype, one incomplete paratype with cirriform branchiae complete, all with black tips. Bipinnate branchiae with lateral branches darker than in complete paratype, resembling those in holotype.
Etymology. The specific epithet is after Dr Helmut Zibrowius, a specialist of serpulid polychaetes and deep-sea ecologist that worked in the Station Marine d’Endoume, Marseille, France, in recognition of his many publications on polychaetes, and especially because he participated in the Musorstom 9 cruise to the Marquesas Archipelago ( Richer de Forges et al. 1999), which included the collection of the type material for this species. The derived name is a noun in the genitive case ( ICZN 1999, Art. 31.1.1) after regarding the last name as Latin.
Remarks. Chloeia zibrowii sp. n. is described with specimens from several localities in the French Polynesia; it belongs in the group viridis because it has a complex pigmentation pattern, and its bipinnate branchiae start in chaetiger 4, becoming progressively smaller posteriorly. It resembles C. hutchingsae sp. n. described above from The Philippines to Australia and Vanuatu, because both species have a well-defined middorsal band, without lateral bands. Additional similarities are found in the size of eyes and type of chaetae. These species differ, however, after their caruncles (pigmentation and number of vertical folds), and in the relative development of bipinnate branchiae, including the number of lateral branches in median segments. In C. zibrowii the caruncle is blackish, with 24 vertical folds, and the branchiae are massive, with 6–7 lateral branches. On the contrary, C. hutchingsae has a pale caruncle, with blackish median ridge, and 34 vertical folds, and its branchiae are delicate, each has 11–12 lateral branches.
Distribution. French Polynesia, in sediments at 260–650 m water depth.
General discussion
Pigmentation patterns.
In two species, specimens can have a pigmentation pattern mostly reddish, against being dark purple or blackish. This was shown for C. viridis from Jamaica, and for C. fusca from Indonesia; the former species was treated elsewhere ( Yáñez-Rivera & Salazar-Vallejo 2022), whereas the latter needs some explanations. Thus, C. fusca was described from Indonesia, and has been confirmed for Papua New Guinea, and distant archipelagos like the French Polynesia, the Loyalty Islands, and Hawai’i. Because the only difference was in the color of the pigmentation pattern (reddish against dark purple or blackish), the different populations were regarded as conspecific, pending a further analysis including molecular or genetic indicators.
A different issue regards to the loss of additional banding, such that only the middorsal spots remain. In at least three colorful species, C. amphora , C. flava and C. pulchella , the additional dorsal bands can fade off completely. This has been regarded as an effect of the specimens being left in ethanol for a long time, some pigment damage due to light, or both. However, there are some videos available in internet showing living specimens with a pale dorsum and only the middorsal spots present ( Iromongara 2009, Senja 2021, 0:00 C. flava , 0:28 + 1:27 C. pulchella ). This would imply either a large, likely clinal variation, or the presence of more than a single biological species. The distribution data are as follows: C. amphora was described from Indonesia and ranges to the Philippine Islands from the intertidal to 45 m depth; C. flava was described from India, and extends to Queensland, Australia, in intertidal to 50 m depth; and C. pulchella was described from northeastern Australia and has been found from India to Japan from the intertidal to 22 m water depth.
Assessing the geographic or depth variation in pigmentation patterns is an interesting research focus for colleagues interested in genetics of pigmentation ( Alvarado 2020, Orteu & Jiggins 2020, vonHoldt et al. 2021), a field that has made spectacular progresses in other colorful marine organisms, especially regarding fishes ( Behrens et al. 2021, Parichi 2021), or benthic invertebrates, including some molluscs (Korshunoa et al. 2020, Neuhaus et al. 2021). On the basis of morphological features, the different populations were regarded as conspecific, pending a further analysis including molecular or genetic indicators. The reason for this perspective is that most marine species are limited to certain geographic areas, and this applies for both, shallow water (Costello et al. 2017, Costello & Chaudhary 2017) and deep-sea species ( Watling et al. 2013).
Species groups. The species of Chloeia can be grouped after several features such as the type of branchiae (pinnate or bipinnate), the start of branchiae (chaetigers 3–5), the dorsal pigmentation pattern (single middorsal bands, single middorsal spots, double longitudinal bands, complex patterns, or none), and some chaetal features. As indicated above, after the combination of these features, the groups are listed in Table 1.
1. Branchiae pinnate (a single species).
2. Branchie bipinnate.
2.1 Branchiae from chaetiger 3 (a single species).
2.2 Branchiae from chaetiger 4.
2.2.1 Branchiae progressively smaller posteriorly
2.2.1.1 Middorsal spots
2.2.1.2 Middorsal bands
2.2.1.3 Complex patterns
2.2.1.4 Dorsum without pigmentation pattern.
2.2.2 Branchiae abruptly smaller after a few chaetigers.
2.3 Branchiae from chaetiger 5.
2.3.1 Middorsal bands
2.3.1.1 Single middorsal bands
2.3.1.2 Double middorsal bands
2.3.2 Dorsum without pigmentation pattern.
It must be kept in mind that for those species having pale pigmentation, or even for those having a colorful body, once the pigments are faded off, they might be included in the colorless groups, but fresh specimens would clarify their correct assignation.
Taxonomic decisions. After the previous information, it must be emphasized that the taxonomic criteria ruling the assignation of specimens to known or undescribed species has depended on a certain combination of morphological features. When a different set of different data is available, as molecular indicators, a different set of conclusions can be reached upon. Of course, some of the conclusions of the future studies might disagree with the current situation, but that is just the way that Science proceeds. Large scale sampling for molecular purposes, especially for those species having a large or very large distribution such as C. flava , C. fusca , C. incerta , or C. pulchella might be especially revealing. Of course, this is not the final study on Chloeia , and the conclusions presented above are just working taxonomic hypothesis.
Symbiosis. The only species that has been recorded as associated with a sessile invertebrate is C. rosea from the Indian Ocean, being found on a subtidal soft coral. The species is also unique by having pinnate, not bippinate, branchiae, but it has not been collected after its original description. It would be useful for future studies that collectors make notes about where the Chloeia specimens were found; most have been regarded as free living, and they are depicted in a few photos or short videos in internet, as indicated above for the different species, but some species might have a closer affinity to other invertebrates. This is another interesting perspective for continuing collecting Chloeia specimens, and for making better observations of what specimens do and what could be their symbionts.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Archinominae |
|
Genus |