Cerithidea weyersi Dautzenberg, 1899
|
publication ID |
https://doi.org/10.11646/zootaxa.3775.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:D9FF6080-0316-4433-ABB8-7D6D6F2BF24B |
|
DOI |
https://doi.org/10.5281/zenodo.5694414 |
|
persistent identifier |
https://treatment.plazi.org/id/03DA0723-6536-2856-D1A0-FE6DFBC68D4B |
|
treatment provided by |
Plazi |
|
scientific name |
Cerithidea weyersi Dautzenberg, 1899 |
| status |
|
Cerithidea weyersi Dautzenberg, 1899 View in CoL
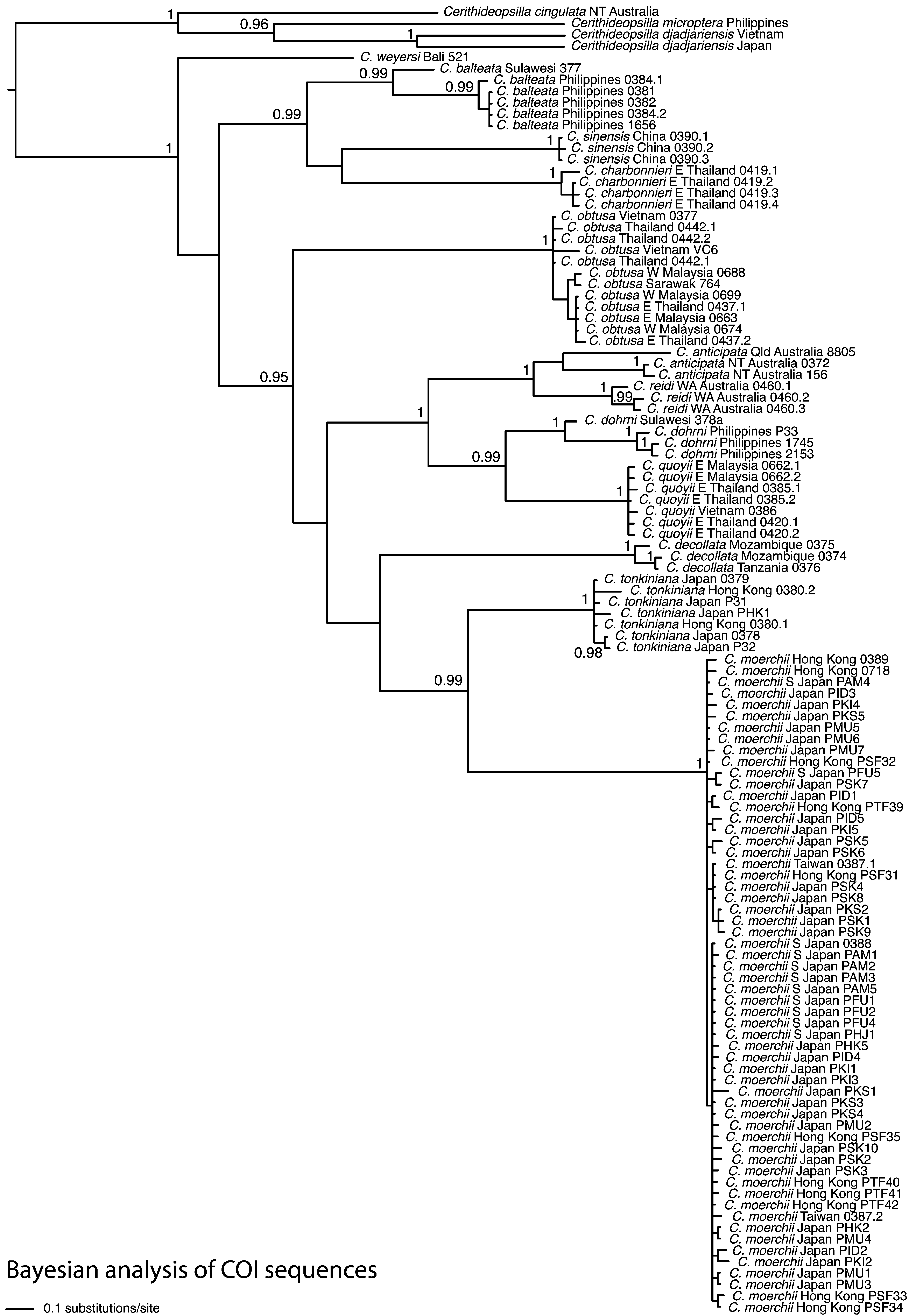
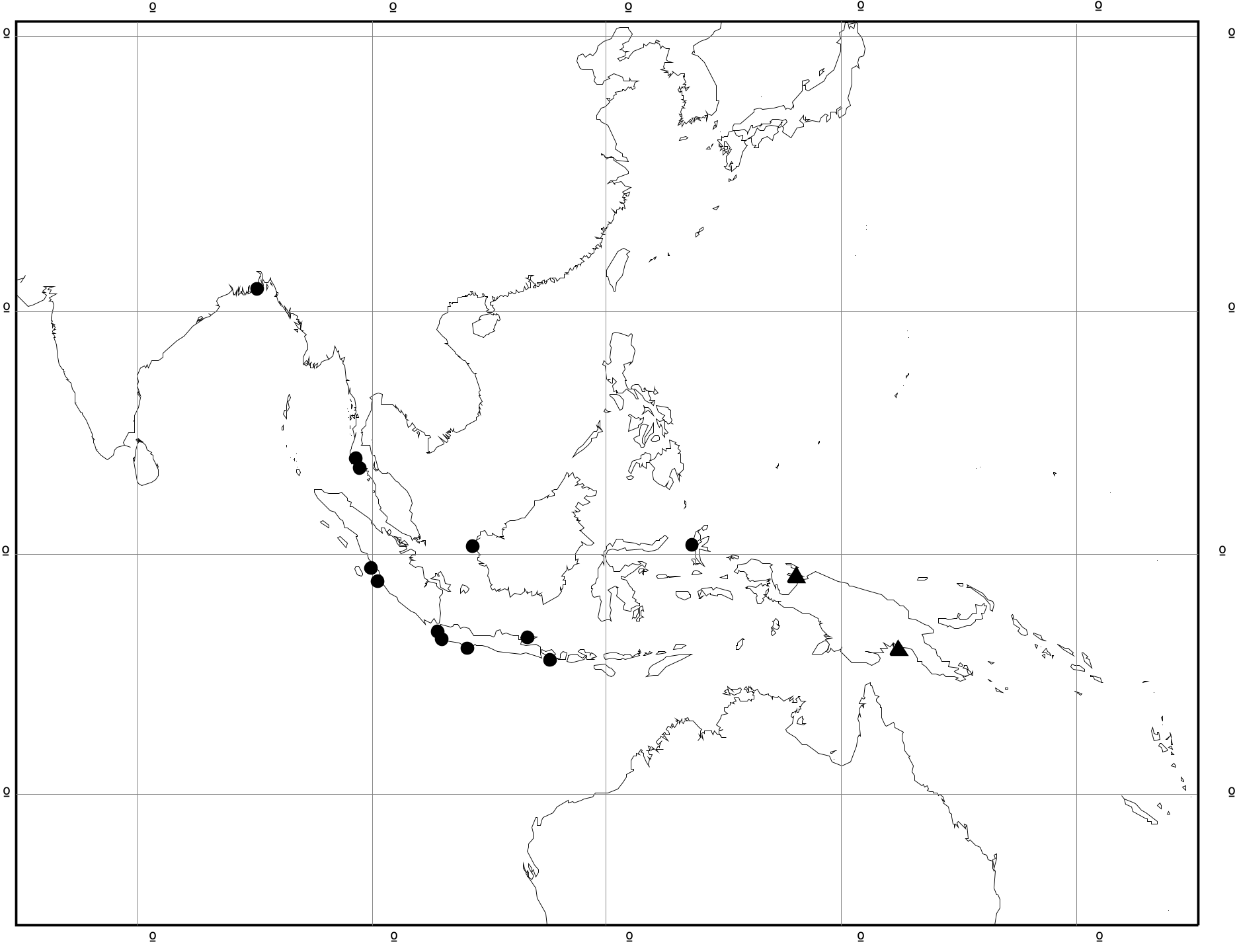
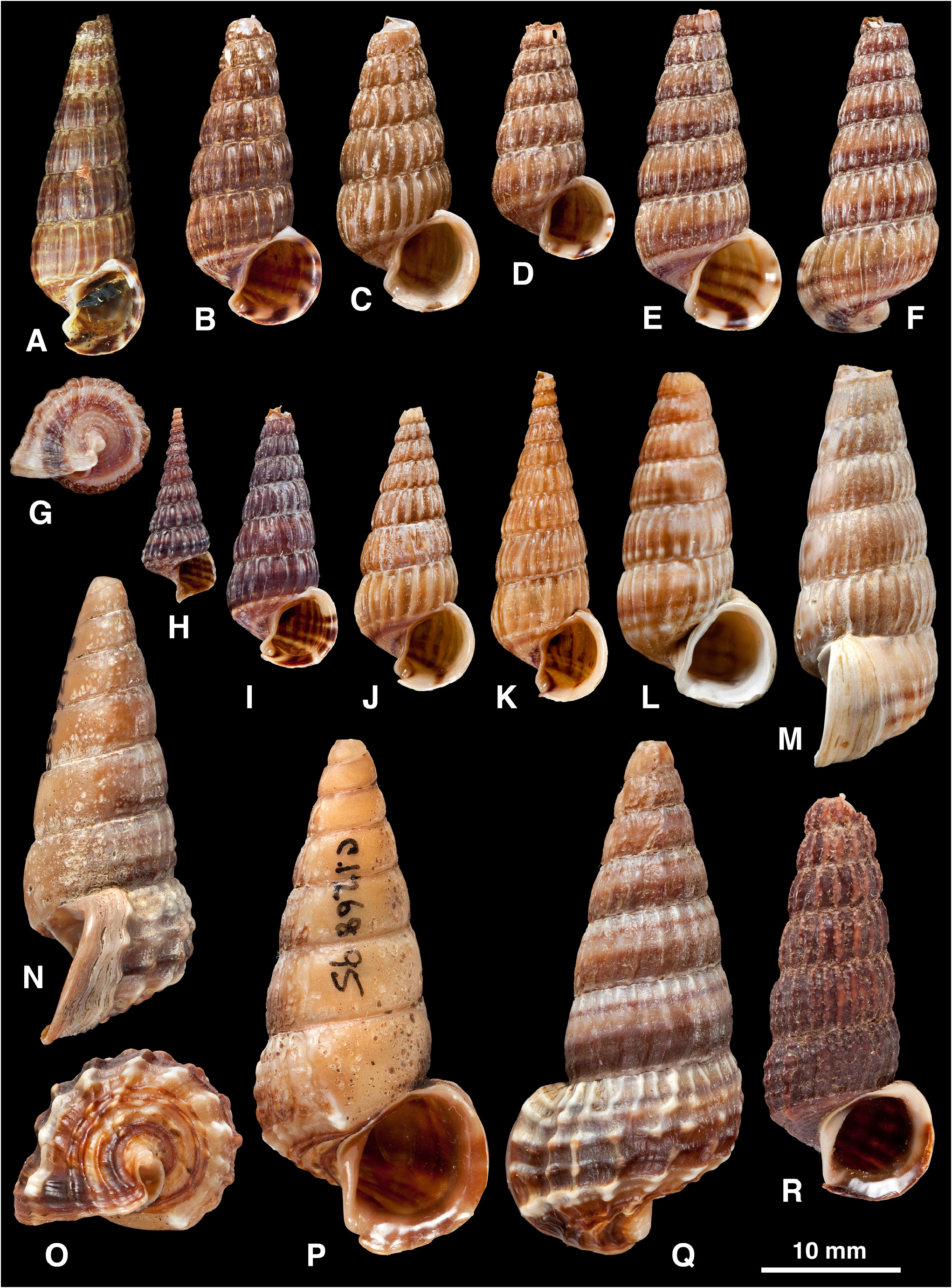
( Figures 2 View FIGURE 2 H, 3A–M, 4)
Cerithidea ornata View in CoL — Boettger, 1890a: 167 (synonymy fide Van Benthem Jutting 1956; not Sowerby, 1855 = C. balteata View in CoL ).
Potamides (Cerithidea) ornatus —von Martens, 1897a: 189 (not Sowerby, 1855; includes C. balteata View in CoL ).
Potamides ornatus — Leschke, 1914: 437 (not Sowerby, 1855).
Cerithidea (Aphanistylus) weyersi Dautzenberg, 1899: 8 View in CoL –10, pl. 2, fig. 1, 1a, 1b (delta de la rivière d’Indrapoera, Sumatra [Indrapura, Indonesia]; 3 syntypes IRSNB MT2772 ( Dautzenberg, 1899: pl. 2, fig. 1, 1a, 1b; Cecalupo 2006: 170, fig.; Fig. 3A View FIGURE 3. A – M ); 3 syntypes IRSNB MT2179; 54 syntypes IRSNB; 55 + 36 probable syntypes IRSNB; 5 syntypes RMNH MOL.175859).
Cerithidea weyersi View in CoL — Van Benthem Jutting, 1956: 436 –438, fig. 105. Dharma, 2005: 92, pl. 21, fig. 7a, b. Mujiono, 2009: 53 – 54, fig. 1F. Reid et al., 2013: figs 1 (shell, phylogeny), 2 (map).
Cerithidea (Cerithidea) weyersi View in CoL — Brandt, 1974: 193 –194, pl. 14, figs 54, 55.
Cerithidea (Cerithideopsis) scalariformis View in CoL — Cecalupo, 2005: 320, pl. 33, fig. 8 (not Say, 1825).
Cerithidea rhizophorarum View in CoL — Cecalupo, 2006: 170, 234, 256, fig. (not A. Adams, 1855).
Cerithidea obtusa View in CoL — Ahmed & Mahmoud, 2007: 10, 95, fig. p. 10 (in part, includes C. obtusa View in CoL ; not Lamarck, 1822).
Cerithidea quadrata View in CoL — Ahmed & Mahmoud, 2007: 96, fig. (not Sowerby, 1866).
Taxonomic history. This species is poorly known and has been widely misidentified. The first misidentification was by Boettger (1890a), whose record of ‘ C. ornata ’ was based on a specimen of C. weyersi from Java, according to Van Benthem Jutting (1956). The reference by von Martens (1897a) is included in the synonymy only because he listed Boettger’s (1890a) record of ‘ C. ornata ’ from Java; the rest of his text applies entirely to C. balteata (his record of ‘ C. ornata ’ from ‘Sumatra’ – where only C. weyersi occurs – is a misquotation of ‘Soemba’ [Sumba] – based on 10 specimens of C. balteata collected by Ten Kate, in RMNH – in Schepman 1892; see Range, below). Following its naming by Dautzenberg (1899), the identity of the species was correctly established by Van Benthem Jutting (1956) and Brandt (1974).
Diagnosis. Shell: small, solid, elongated-pupoidal with expanded aperture, periphery rounded; 18–30 rounded axial ribs (remaining strong throughout final whorl); ventrolateral varix absent or weak, at 230–270°; no spiral sculpture above periphery; often a strong pattern of spiral lines. Eastern Indian Ocean, Java, Borneo, Moluccas. COI GenBank HE680298 View Materials .
Material examined. 26 lots.
Shell ( Fig. 3A–M View FIGURE 3. A – M ): H = 15.8–33 mm. Shape elongated-pupoidal (H/B = 1.91–2.43, SH = 2.87–3.00); decollate, 5–7 whorls remaining; spire whorls slightly rounded; spire profile convex, concave at attenuated apex if only partially decollate ( Fig. 3H, K View FIGURE 3. A – M ); periphery rounded; relatively solid. Adult lip moderately flared and thickened, sometimes greatly thickened (e.g. 6.2 mm thick in shell H = 24.6 mm; Fig. 3M View FIGURE 3. A – M ); rarely 1–2 previous lips on last whorl; apertural margin planar in side view; weak anterior projection adjacent to canal. Sculpture on spire of straight to slightly opisthocline axial ribs, becoming slightly curved (opisthocyrt) on final 1–3 whorls, remaining strong on final whorl and continuing weakly on base, ribs rounded, ribs and interspaces of equal width, 18–30 ribs on penultimate whorl; spiral sculpture absent above periphery; base with 9–11 indistinct striae. Ventrolateral varix absent or weakly developed at 230–270°. Surface with faint, fine spiral microstriae on periostracum, satin sheen. Colour: fawn, usually a brown band just below periphery and brown line adjacent to columella, usually 3–4 brown lines above periphery; occasionally without lines ( Fig. 3J View FIGURE 3. A – M ) or lines may fuse so shell is entirely brown above periphery ( Fig. 3I View FIGURE 3. A – M ); periostracum pale to dark brown; bands visible within aperture.
Animal ( Fig. 2 View FIGURE 2 H): Fawn, mottled with brown; 3 brown to black bands across snout, becoming darker distally; tentacles with transverse black lines, black band across eye; sides of foot mottled with grey, brown and orange brown; mantle edge fawn.
Range ( Fig. 4 View FIGURE 4 ): Bangladesh, W Thailand, W Sumatra, Java, Borneo, Moluccas. Records: Bangladesh: Char Kukrimukri, Bhola ( NHMUK 20130231); R. Ganges ( ZMB). Thailand: Ban Nai Sa, Krabi Prov. ( RMNH); Kantang, Trang Prov. ( NHMUK 20130230; RMNH; AM; USNM 777194; ANSP 330948). Indonesia: Mempawah, Kalimantan ( ZMB); Marang, Sumatra ( ANSP 72450); Indrapoera, Sumatra ( RMNH); Welkomst Bay, Banten, Java ( USNM 261761); Anyer–Labuhan road, Java ( ZMB 106.427); Tji Mandiri estuary, Java ( RMNH); Pangandaran– Cipatujah road, Java ( ZMB 106.428); S Madura, Java ( RMNH); W Gumbrih, Bali ( ZMB 191.521); Ternate, Halmahera ( RMNH 175855).
The records are mostly from the western and southern edge of continental Southeast Asia and the former Sundaland, but with two exceptions (listed above). One, from western Borneo, is a single shell collected at Mempawah by von Martens in the late nineteenth century and is considered reliable. The other is from Halmahera, based on eleven specimens collected in 1914 by E.F. Jochim. The identification is not in question but, since there are no records between this locality and Bali, the occurrence requires confirmation.
In addition to the known range in Thailand, Sumatra and Java, Brandt (1974) included Buru, Sumba and Timor in eastern Indonesia, and Luzon and Negros in the Philippines, in the distribution of this species. These last five records were listed without authority and appear to have been taken from an identical list of localities given by von Martens (1897a) for C. ornata [= C. balteata ]. In this, Brandt appears to have followed Van Benthem Jutting’s (1956) inclusion of von Martens’ ‘ C. ornata ’ in the synonymy of C. weyersi . Nevertheless, von Martens’ text clearly applies largely to C. balteata (see Taxonomic History, above). Therefore, the only known record of C. weyersi from the Banda or Molucca Seas is that from Halmahera, mentioned above.
Habitat and ecology. Habitats recorded on museum labels include: “Nipa-palm swamp” in Trang Prov., Thailand (leg. R.A.M. Brandt, NHMUK 20130230); “river 200 m from sea” in Java (leg. T. von Rintelen, ZMB); “bases of Sonneratia trees in mangrove plantation” in Bangladesh (leg. L.M. Cook, NHMUK 20130231). At Gumbrih, Bali, it was collected on the banks of a short lowland river, at a site with brackish (but seasonally fresh) water, together with freshwater cerithioideans ( Stenomelania , Tarebia , ‘ Thiara ’ scabra) (M. Glaubrecht, pers. comm.). In Java, Van Benthem Jutting (1956) recorded C. weyersi from river estuaries that were brackish at high tide and fresh at low tide, while Mujiono (2009) reported it on roots and trunks of mangroves. In Bangladesh it has been said to climb on trees, but a figure shows it attached to grass ( Ahmed & Mahmoud 2007: 10, 96). In Krabi Prov., Thailand, it was found on vegetation at the margin of a fast-flowing river, shaded by mangrove trees, at the upstream limit of brackish influence (B. Craig, pers. comm.).
Remarks. This is a little known species, poorly represented in museum collections. Its habitat is apparently towards the freshwater end of the estuarine range and occasional large samples suggest that it can sometimes be abundant. Possibly, this transitional habitat might explain why it has seldom been collected, since it does not occur in typically marine mangrove environments or in fully freshwater streams. Its distribution is peculiar. The majority of the records are from the eastern margin of the Indian Ocean ( Bangladesh, W Thailand, Sumatra, Java and Bali), an area that corresponds with the margin of Sundaland during glacial low sea-level stands ( Voris 2000). This implies that the distribution could be relictual, because of limited ability to recolonize the shelf when sea levels rose in the Holocene. However, the records from Borneo and Halmahera do not support this. The recorded distribution is likely to be incomplete.
In a molecular phylogeny based only on mitochondrial COI sequences ( Reid et al. 2013; Fig. 1 View FIGURE 1 ), the single individual of C. weyersi forms one of three basal branches in the genus. Morphological comparison suggests that it is sister to C. houbricki , and their distinction is discussed in the Remarks on that species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Family |
|
|
Genus |
Cerithidea weyersi Dautzenberg, 1899
| Reid, David G. 2014 |
Cerithidea obtusa
| Ahmed 2007: 10 |
Cerithidea quadrata
| Ahmed 2007: 96 |
Cerithidea rhizophorarum
| Cecalupo 2006: 170 |
Cerithidea (Cerithideopsis) scalariformis
| Cecalupo 2005: 320 |
Cerithidea (Cerithidea) weyersi
| Brandt 1974: 193 |
Cerithidea weyersi
| Mujiono 2009: 53 |
| Dharma 2005: 92 |
| Van 1956: 436 |
Potamides ornatus
| Leschke 1914: 437 |
Cerithidea (Aphanistylus) weyersi
| Cecalupo 2006: 170 |
| Dautzenberg 1899: 8 |
Potamides (Cerithidea) ornatus
| Martens 1897: 189 |
Cerithidea ornata
| Boettger 1890: 167 |