Cerithidea balteata A. Adams, 1855
|
publication ID |
https://doi.org/10.11646/zootaxa.3775.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:D9FF6080-0316-4433-ABB8-7D6D6F2BF24B |
|
DOI |
https://doi.org/10.5281/zenodo.5694418 |
|
persistent identifier |
https://treatment.plazi.org/id/03DA0723-652B-2850-D1A0-FAD0FC488FE2 |
|
treatment provided by |
Plazi |
|
scientific name |
Cerithidea balteata A. Adams, 1855 |
| status |
|
Cerithidea balteata A. Adams, 1855 View in CoL
( Figures 5 View FIGURE 5 , 6 View FIGURE 6 )
Cerithidea balteata A. Adams, 1855: 84 View in CoL
(Island of Ticao [ Philippines]; lectotype here designated NHMUK 20130222/1, Fig. 5 View FIGURE 5 A, 2 paralectotypes NHMUK 20130222/2, 3).
Hidalgo, 1904 –1905: 202–203. Reid et al., 2013: figs 1 (shell, phylogeny), 2 (map).
Cerithium (Cerithidea) balteatum View in CoL — Kobelt, 1895: 177, pl. 33, figs 5, 6 ( Cecalupo 2005: pl. 31, fig. 11).
Cerithidea balteata View in CoL —Casto de Elera, 1896: 329.
Cerithidea raricostata A. Adams, 1855: 85 View in CoL
(“Catbalonga, island of Samaar” here corrected to Calbayog, Samar [ Philippines], as per original label; 3 syntypes NHMUK 20130223, seen).
Cerithium ( balteata var.?) rarecostatum View in CoL — Kobelt, 1895: 178–179, pl. 33, figs 7, 8 ( Cecalupo 2005: pl. 31, fig. 12).
Cerithidea (Aphanistylus) raricostata View in CoL —Casto de Elera, 1896: 330.
Cerithidea raricosta — Hidalgo, 1904 –1905: 203.
Cerithidea cornea A. Adams, 1855: 85 View in CoL
(“Borneo” here corrected to Guimaras [ Philippines], as per original label; 3 syntypes NHMUK 20130224, Fig. 5 View FIGURE 5 D, seen).
Sowerby, 1866: sp. 21, pl. 3, fig. 21. Casto de Elera, 1896: 328.
Cerithium corneum — Sowerby, 1855: 887, pl. 186, fig. 275.
Potamides (Cerithidea) corneus — Tryon, 1887: 162, pl. 32, fig. 55 (as cornea View in CoL ). von Martens, 1897a: 190.
Cerithium (Cerithidea) corneum — Kobelt, 1890a: 50 –51, pl. 10, figs 6, 7 ( Cecalupo 2005: pl. 31, fig. 5).
Cerithium ornatum ‘A. Adams’ G.B. Sowerby II, 1855 : 887, pl. 186, figs 277, 278
(“ Philippines ”, here corrected to Ticao, Philippines, as per original label; lectotype here designated NHMUK 20130222/1, Fig. 5 View FIGURE 5 A, Sowerby, 1855: pl. 186, fig. 278, 2 paralectotypes NHMUK 20130222/2, 3; 2 paralectotypes NHMUK 20130225,
Sowerby, 1855: pl. 186, fig. 277).
Cerithidea ornata View in CoL — Sowerby, 1866: sp. 22, pl. 4, fig. 22a, b. Casto de Elera, 1896: 328. Hidalgo, 1904 –1905: 203. Schepman, 1909: 168 –169. Oostingh, 1925: 50. Lozouet, 2008: 284, pl. 87, figs 7, 8. Lozouet & Plaziat, 2008: 57, 112, pl. 20, figs 7– 9. Reid et al., 2008: 680 –699, figs 1, 2 (phylogeny) (in part, includes C. tonkiniana View in CoL ).
Potamides (Cerithidea) ornata — Tryon, 1887: 162, pl. 32, fig. 64. von Martens, 1897a: 189 (in part, includes C. weyersi fide Van Benthem Jutting 1956 ; as ornatus ).
Cerithium (Cerithidea) ornatum — Kobelt, 1890a: 51 –52, pl. 11, figs 1, 2 ( Cecalupo 2005: pl. 31, fig. 8).
Cerithium (Cerithidea) quadrasi Kobelt, 1895: 176 , pl. 33, figs 3, 4
(Marinduque, Philippines; 2 syntypes SMF 228128, photograph seen, 228129; Cecalupo 2005: pl. 31, fig. 9) .
Cerithium (Cerithidea) balteatum var. mindorensis Kobelt, 1895: 179 View in CoL , pl. 33, fig. 9
(Mindoro [ Philippines]; holotype SMF 228127, photograph seen; Cecalupo 2005: pl. 32, fig. 28).
Cerithidea (Cerithideopsis) scalariformis View in CoL — Cecalupo, 2005: 316–318, pl. 31, figs 5, 9, 11, 12, pl. 32, fig. 28 (not Say, 1825; in part, includes Cerithideopsis scalariformis View in CoL ). Cecalupo, 2006: 34, 53, 102, 133, 179, 190, 218, 233, 236, 237 (not Say, 1825; in part, includes Cerithideopsis scalariformis View in CoL ).
Cerithidea anticipata View in CoL — Cecalupo, 2005: 316, pl. 31, fig. 8 (not Iredale, 1929; in part, includes C. anticipata View in CoL ). Cecalupo, 2006: 118, 176, 224 (not Iredale, 1929; in part, includes C. anticipata View in CoL , Cerithideopsilla conica , Cerithideopsis largillierti View in CoL ).
Taxonomic history. This species is poorly known and variable in appearance, accounting for its complex history. Four of the six available names were introduced in 1855 ( C. balteata , C. raricostata and C. cornea by Adams; Cerithium ornatum by Sowerby). None is well known, but Cerithium ornatum has been used a little more frequently. However, Cerithium ornatum has far more often been used in an incorrect sense (for C. tonkiniana ). As first reviser, I choose C. balteata for the present species, to minimise confusion (this name was also used recently for this species by Reid et al. 2013). Adams’ paper was published on 11 April 1855 and although the exact date of Sowerby’s work is not known, it can be assumed to have been later ( Petit 2009) because he uses most of Adams’ new names and copied some of his descriptions. (In fact Sowerby 1855: 848 mentions only the use of a manuscript by Adams and therefore may not have seen Adams’ published monograph of Cerithidea ). Sowerby did not, however, mention C. balteata . Instead he introduced Cerithium ornatum and attributed the name to Adams. One of Sowerby’s figured specimens ( Sowerby 1855: pl. 186, fig. 278; here designated lectotype of Cerithium ornatum ) is part of the type series of C. balteata . Sowerby assigned most cerithioideans to the genus Cerithium , so Adams’ species would have become a junior homonym of Cerithium balteatum Philippi, 1848 . While this was probably the reason why Sowerby introduced the name Cerithium ornatum , he did not explicitly do so as a replacement for C. balteata . Furthermore, the description and type locality are not exactly the same as those of C. balteata (unlike, for example, Sowerby’s text for Adams’ species C. cornea , which is an exact copy of Adams 1855), and the type material includes additional specimens. For these reasons Cerithium ornatum is regarded as a new species, not a replacement name. To remove any ambiguity, the same specimen is here designated lectotype of each, so that the names become objective synonyms. Authorship of Cerithium ornatum is here attributed to Sowerby (not ‘Adams in Sowerby’ as per Petit 2009: 34, 147), because of the discrepancies between the descriptions of C. balteata and Cerithium ornatum , and because the renaming of Adams’ species was only necessary because of its classification in the broad genus Cerithium as advocated by Sowerby, whereas Adams himself used Cerithidea ( Adams 1855) .
The type locality of C. cornea was given as “Borneo, mouths of rivers” ( Adams, 1855), and this was repeated by Sowerby (1855, 1866). However, the original label accompanying the syntypes, written by the collector Hugh Cuming, reads “Isle of Guimaras in marshy places overflowed by the sea at times”. There is no firm evidence that this species occurs on the mainland of Borneo, so the type locality is here corrected.
The number of synonyms reflects the variability of this species in colour, thickness and number of axial ribs. Cerithidea quadrasi appears to be a form with strong spiral lirae. A species from the Far East, C. tonkiniana , has been widely confused with the present species, under the name C. ornata . Cecalupo (2005, 2006) synonymized Cerithium ornatum with C. anticipata , and all the other synonyms listed above with the western Atlantic species Cerithideopsis scalariformis .
Diagnosis. Shell: periphery rounded or slightly angled; aperture thickened and flared; 8–31 rounded axial ribs on penultimate whorl, 0–6 weaker ribs after ventrolateral varix; ventrolateral varix a strong or inconspicuous rib at (180)210–280°; no spiral sculpture on spire but 1–6 weak ridges above periphery on last whorl; often strongly banded or lined pattern. Philippines, Moluccas, New Guinea, Solomon Is. COI GenBank AM932830 View Materials , HE680208 View Materials , HE680225 View Materials – HE680228 View Materials .
Material examined. 111 lots.
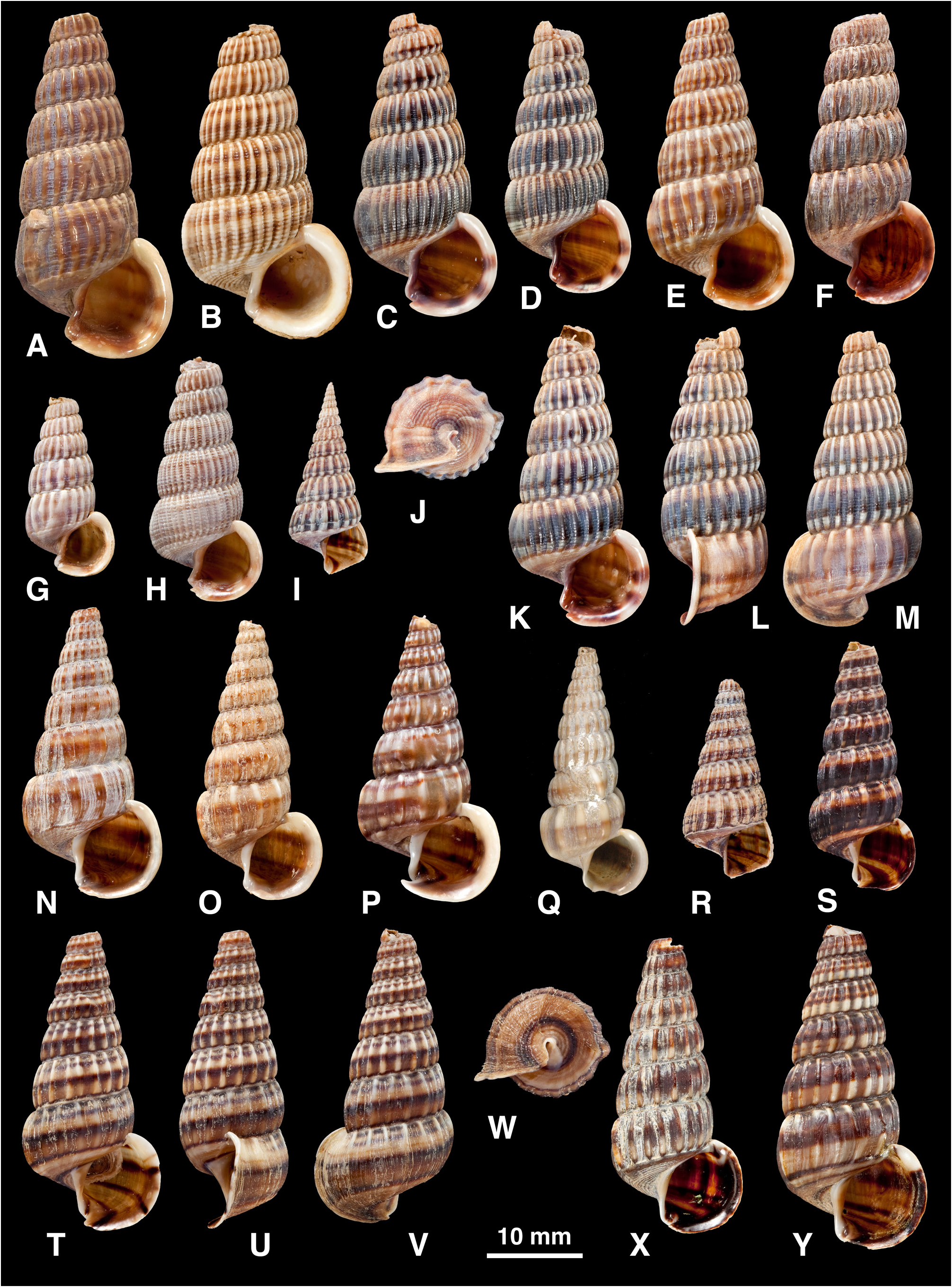
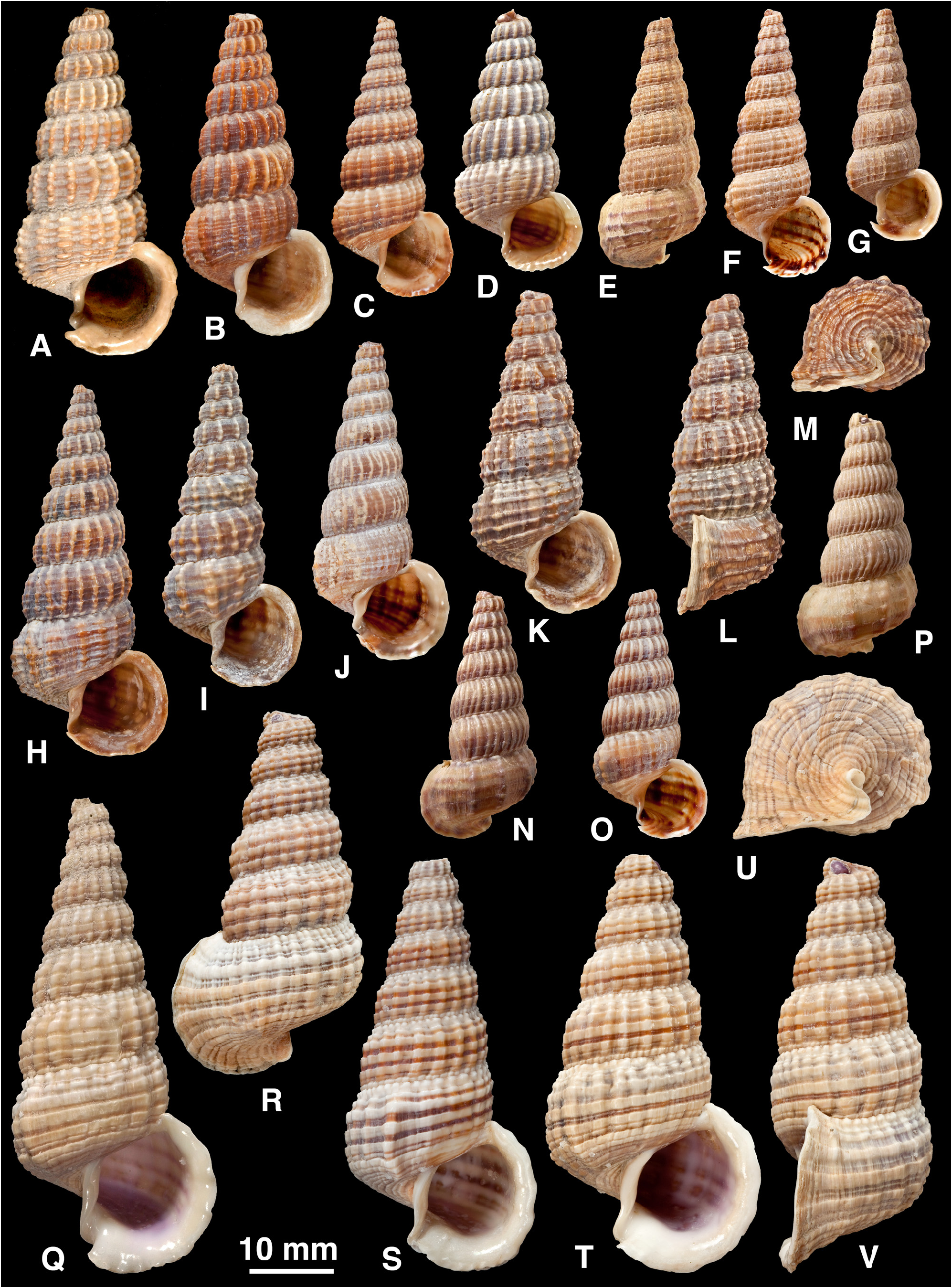
Shell ( Fig. 5 View FIGURE 5 ): H = 16.0– 37.3 mm. Shape elongated conical (H/B = 1.91–2.37, SH = 2.46–3.60); decollate, 5– 8 whorls remaining; spire whorls rounded to flattened, suture distinct; spire profile straight to slightly convex; periphery rounded to slightly angled; thin to moderate thickness. Adult lip flared, moderately thickened; occasionally a previous lip on final whorl; apertural margin planar in side view; moderate anterior projection adjacent to canal. Sculpture on spire of straight to slightly opisthocline axial ribs, becoming slightly curved (opisthocyrt) on final 1–2 whorls, ribs usually prominent, rounded, interspaces1–2 times width of ribs, 8–31 ribs on penultimate whorl; axial ribs after ventrolateral varix usually weaker and more distant than on spire, or irregularly rugose, numbering 0–6; spiral sculpture absent on spire, but 1–6 weak ridges often develop above periphery on final whorl (rarely, ridges conspicuous between ribs on last 2–4 whorls; Fig. 4 View FIGURE 4 Q–U); base with 5–10 striae, of which peripheral one may form weak keel. Ventrolateral varix usually a thickened rib at (180)210–280°, but may be inconspicuous or absent. Surface with faint, fine spiral microstriae on periostracum; ribs shiny when worn. Colour: fawn, usually 1–6 dark brown bands or lines above periphery and 2 on base, sometimes dark brown with indistinct pale bands; bands show through in aperture.
Animal: Head grey with pink and pale yellow spots; snout grey with transverse black lines and 3 black bands, tip yellow; tentacles grey with black transverse lines, black band across eye, pink base behind eye; sides of foot pinkish grey with dark grey mottling; mantle grey, edge cream (based on ethanol-preserved specimens from Sulawesi).
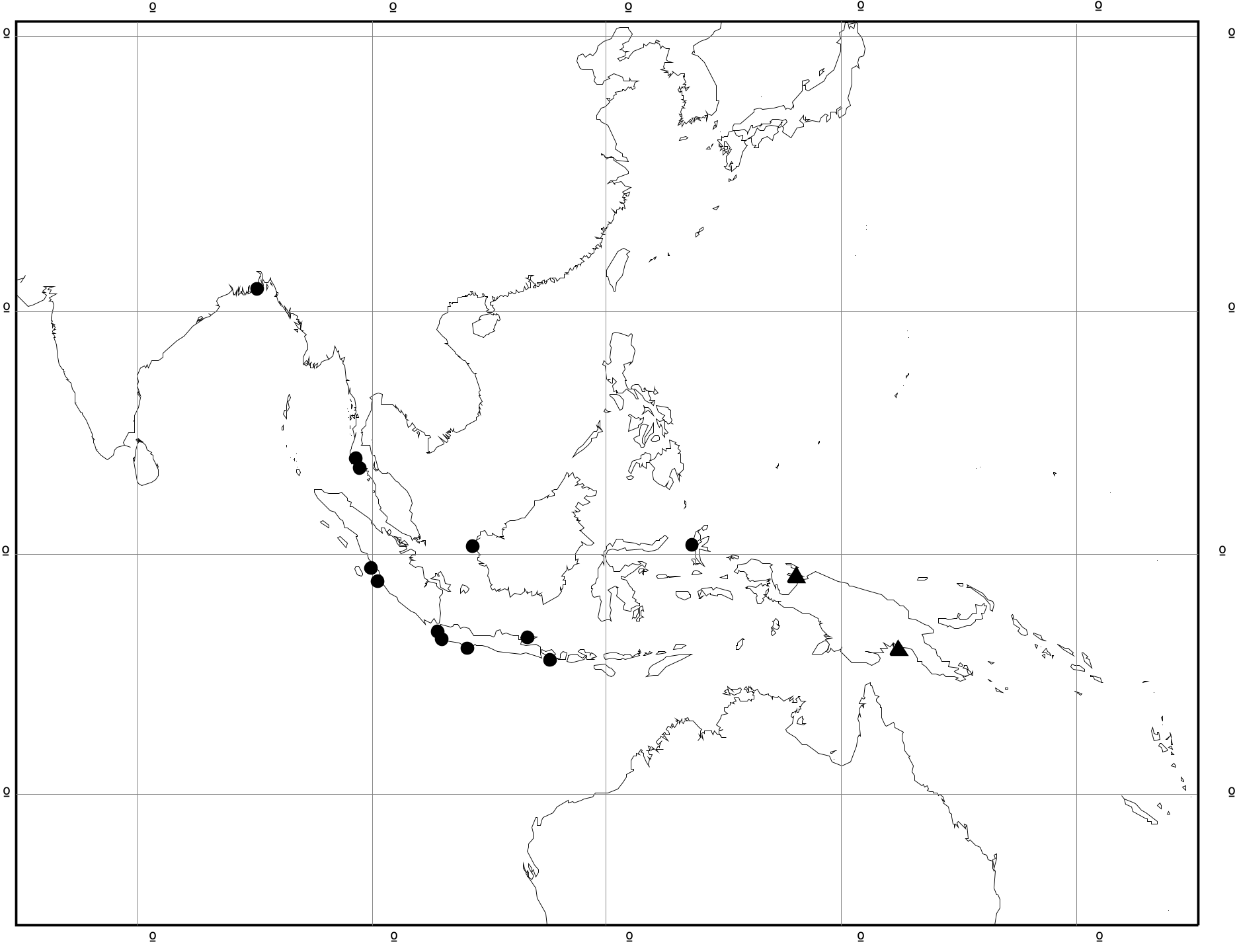
Range ( Fig. 6 View FIGURE 6 ): Philippines, Sulawesi, Moluccas, Lesser Sunda Is, New Guinea, Solomon Is. Records: Philippines: Port San Vicente, Palaui I., Luzon ( USNM 259402); Malinao, Albay, Luzon ( USNM 1165); Malubatglubut I., Palawan ( USNM 233037); Port Dupon, Leyte ( USNM 232971); Jordan R., Guimaras ( USNM 243887); Port Tataan, Tataan I. ( USNM 862783); Surigao, Mindanao ( USNM 544065); Davao, Mindanao ( USNM 233133). Indonesia: Manado, Sulawesi ( NHMUK); Luwuk, Sulawesi ( ZMB 191.377); Tembecha, Kendari, Sulawesi ( ZMB 191.376); Lovina, Bali ( ZMB 106.180); Waiara, Maumere, Flores ( ZMB 106.573); Kupang, Timor ( ZMB); Bacan, Moluccas ( RMNH); Obi, Moluccas ( NHMUK 1903.4.863; AM C.33594; RMNH); Aru Is ( NHMUK 1858.3.17.29); Warir I., E of Salawati I., East Papua ( RMNH); Mimika R., Kokenau, East Papua (AM C.116356); Sorendidori, E side Soepiori I., Schouten Is, East Papua ( ANSP 207473). Solomon Is: Shortland Is ( ANSP 327646); Aola, Guadalcanal ( NHMUK 1952.1.4.150; ZMB); Santa Cruz Is (AM C.29754).
Occurrence in Kalimantan (Indonesian Borneo) is unconfirmed, but there are two samples in ZMB with untraceable localities in ‘East Borneo’ or ‘Southeast Borneo’.
Habitat and ecology. In Bohol, Philippines, it has been reported to climb on Nypa palms in the middle and upper estuary of the Abatan River (salinity 2–10 ppt) and to be replaced by C. dohrni where salinity rises above 10 ppt ( Lozouet & Plaziat 2008). It was collected by von Martens (1897a) in slightly salty swamps with Neritina and Neritodryas , and in brackish lagoons on the trunks of the mangrove Sonneratia .
Remarks. Shell morphology is highly variable and four distinctive regional forms can be distinguished. There is some apparent intergradation between forms, but their ranges are separated by either open-water gaps or regions without adequate sampling (e.g. northern New Guinea and southern Banda Sea):
(1) Philippines ( Fig. 5 View FIGURE 5 A–K, V, W): whorls rounded; axial ribs strong and close-set; often 1–2 dark brown bands on spire contrasting with whitish ribs. This is the typical form, on which C. balteata and all its synonyms are based.
(2) Sulawesi, Moluccas, Lesser Sunda Is ( Fig. 5 View FIGURE 5 L–P, CC, DD): whorls more flattened; axial ribs strong and closeset; often a pattern of 4–5 brown lines above periphery.
(3) Western New Guinea and Aru Is ( Fig. 5 View FIGURE 5 Q–U): shell thin; axial ribs weak, narrow and irregular; spiral ridges present between ribs on final 2–4 whorls; often a single broad brown band above periphery.
(4) Solomon Is ( Fig. 5 View FIGURE 5 X–BB): shell thick; axial ribs strong but sparse and remaining well developed after ventrolateral varix; usually dark brown.
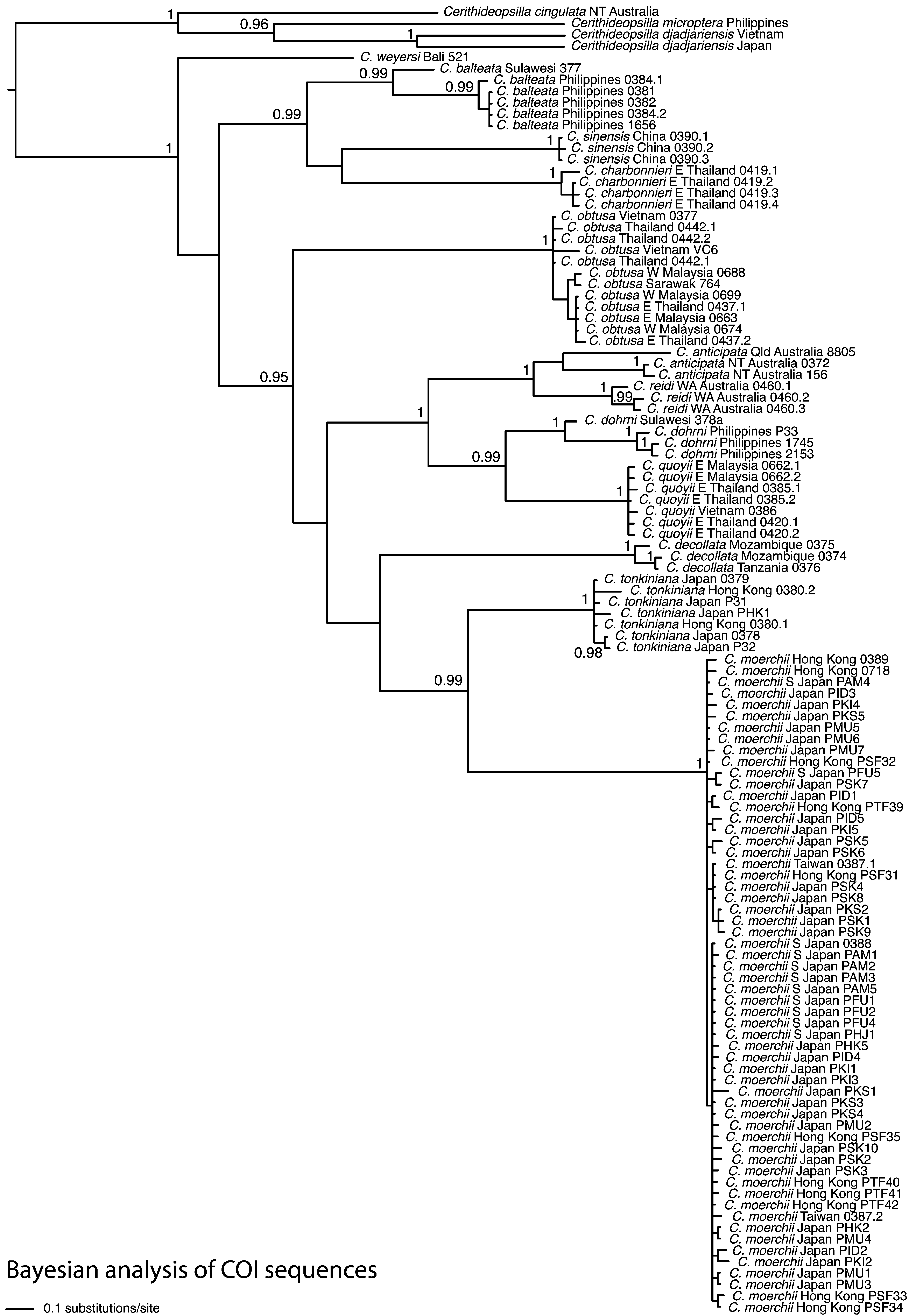
A single specimen from Luwuk, Sulawesi (ZMB 191.377) had a mean uncorrected pairwise distance for COI of 0.05 from specimens from the Philippines (close to the minimum between recognized species, 0.072 for the pair C. anticipata and C. reidi, Reid et al. 2013 ; Fig. 1 View FIGURE 1 ), suggesting that at least forms (1) and (2) could perhaps be distinct species. The geographical range is very wide for a single species of Cerithidea and spans several range endpoints and recognized phylogeographic boundaries between sister species in the genus, which could also point to this being a species complex. On the other hand, the range extends across all the islands of eastern Indonesia and the species (or complex) has reached the Solomon Islands, unlike any other member of the genus; this could imply a wider larval dispersal, and consequently wider range, than in other congeners. Further study with denser sampling of this widespread and variable species is required.
The distribution of this species covers the Philippines, eastern Indonesia, northwestern New Guinea and the Solomon Is, which are all areas of low oceanic primary productivity. Purely on the basis of this geographical pattern, this species could be described as more ‘oceanic’ than its congeners, which show typically ‘continental’ distribution patterns. This distinction has been emphasized among littorinid gastropods (e.g. Echinolittorina ; Williams & Reid 2004; Reid et al. 2006) and may relate to an aspect of the ecology of planktotrophic larvae. Oceanic littorinids typically show wider distributions and extend further across the Pacific Plate than their continental congeners, and C. balteata also does so. However, C. balteata also occurs in southwestern New Guinea on the Sahul Shelf, which is an area of high oceanic productivity, so the value of the oceanic/continental distinction in this genus is uncertain.
In molecular phylogenies based on the COI and 28S rRNA genes ( Reid et al. 2013; Fig. 1 View FIGURE 1 ), this species forms a well-supported clade together with C. sinensis and C. charbonnieri . These three species should not, however, be confused: C. sinensis has a sinuous apertural margin, no peripheral keel and no ventrolateral varix; C. charbonnieri has a sinuous apertural margin, a sharp peripheral keel and a ventrolateral varix; C. balteata has a planar and expanded aperture, a moderate peripheral cord and usually a ventrolateral varix ( Figs 5 View FIGURE 5 , 7 View FIGURE 7. A – L ). Their geographical distributions do not overlap.
This species has been widely confused with C. tonkiniana (both being combined under the name ‘ C. ornata ’). Although their distributions do not overlap ( C. tonkiniana occurring in southern Japan, China and Vietnam) and they are not phylogenetically close ( Fig. 1 View FIGURE 1 ), their shells are extremely similar. That of C. balteata is more variable, with 8–31 axial ribs on the penultimate whorl, a ventrolateral varix usually at 210–280° and no spiral sculpture on the upper spire whorls ( Fig. 5 View FIGURE 5 ). The shell of C. tonkiniana is slightly broader and more solid, has 11–25 axial ribs on the penultimate whorl, a varix usually at 180–220° and (if not eroded, which most are) shows 5 weak spiral ridges on the spire ( Fig. 13 View FIGURE 13. A – M N–Y).
In the western half of New Guinea, C. balteata occurs together with C. anticipata . Typical members of both species are easily separated by the lack of spiral sculpture in C. balteata . However, in the area of sympatry their discrimination can be difficult, because the local form of C. balteata does have spiral sculpture; this is usually relatively weak, the ridges are slightly irregularly spaced and do not cross the strong axial ribs ( Fig. 5 View FIGURE 5 Q–U). In contrast, the spiral ridges of C. anticipata are stronger, more regularly spaced and cross the weaker axial ribs to give a cancellate effect ( Fig. 10E–G View FIGURE 10. A – P ). In addition, shells of C. balteata are a little narrower, more cylindrical and are more likely to show a previous apertural lip on the body whorl. Available data suggest that C. balteata occupies habitats of reduced salinity, whereas C. anticipata occurs in fully marine habitats; this distinction is likely to be maintained in regions of sympatry.
This species is not collected for food in Bohol ( Lozouet & Plaziat 2008).
| NHMUK |
Natural History Museum, London |
| SMF |
Forschungsinstitut und Natur-Museum Senckenberg |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| ZMB |
Museum für Naturkunde Berlin (Zoological Collections) |
| RMNH |
National Museum of Natural History, Naturalis |
| ANSP |
Academy of Natural Sciences of Philadelphia |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Family |
|
|
Genus |
Cerithidea balteata A. Adams, 1855
| Reid, David G. 2014 |
Cerithidea (Cerithideopsis) scalariformis
| Cecalupo 2006: 34, 53, 102, 133, 179, 190, 218, 233, 236, 237 |
| Cecalupo 2005: 316-318 |
Cerithidea anticipata
| Cecalupo 2006: 118, 176, 224 |
| Cecalupo 2005: 316 |
| Reid, D. G., Claremont, M., Smith, L., Shamoto, M., Glaubrecht, M. & Ozawa, T. 2013: 18 |
| Hidalgo, J. G. 1904: 202 |
Cerithidea raricosta
| Hidalgo, J. G. 1904: 203 |
Cerithidea balteata
| Elera 1896: 329 |
Cerithidea (Aphanistylus) raricostata
| Elera 1896: 330 |
| Elera 1896: 328 |
Cerithidea ornata
| Lozouet 2008: 57 |
| Reid 2008: 680 |
| Oostingh 1925: 50 |
| Schepman 1909: 168 |
| Elera 1896: 328 |
Cerithium (Cerithidea) balteatum
| Kobelt 1895: 177 |
Cerithium ( balteata var.?) rarecostatum
| Kobelt 1895: 178-179 |
Cerithium (Cerithidea) quadrasi
| Kobelt 1895: 176 |
Cerithium (Cerithidea) corneum
| Kobelt 1890: 50 |
Cerithium (Cerithidea) ornatum
| Kobelt 1890: 51 |
Potamides (Cerithidea) corneus
| Martens 1897: 190 |
| Tryon 1887: 162 |
Potamides (Cerithidea) ornata
| Martens 1897: 189 |
| Tryon 1887: 162 |
Cerithidea balteata
| Adams 1855: 84 |
Cerithidea raricostata
| Adams 1855: 85 |
Cerithidea cornea
| Adams 1855: 85 |
Cerithium corneum
| Sowerby 1855: 887 |
Cerithium ornatum ‘A. Adams’ G.B. Sowerby II, 1855
| Sowerby 1855: 887 |