Caiman crocodilus apaporiensis
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4059.3.6 |
|
publication LSID |
lsid:zoobank.org:pub:A23EEA5E-1351-40F3-93ED-C3DFED51294E |
|
DOI |
https://doi.org/10.5281/zenodo.6104010 |
|
persistent identifier |
https://treatment.plazi.org/id/03891D31-FFF0-FFBC-FF6C-64A5FAC376F4 |
|
treatment provided by |
Plazi |
|
scientific name |
Caiman crocodilus apaporiensis |
| status |
|
Caiman crocodilus apaporiensis
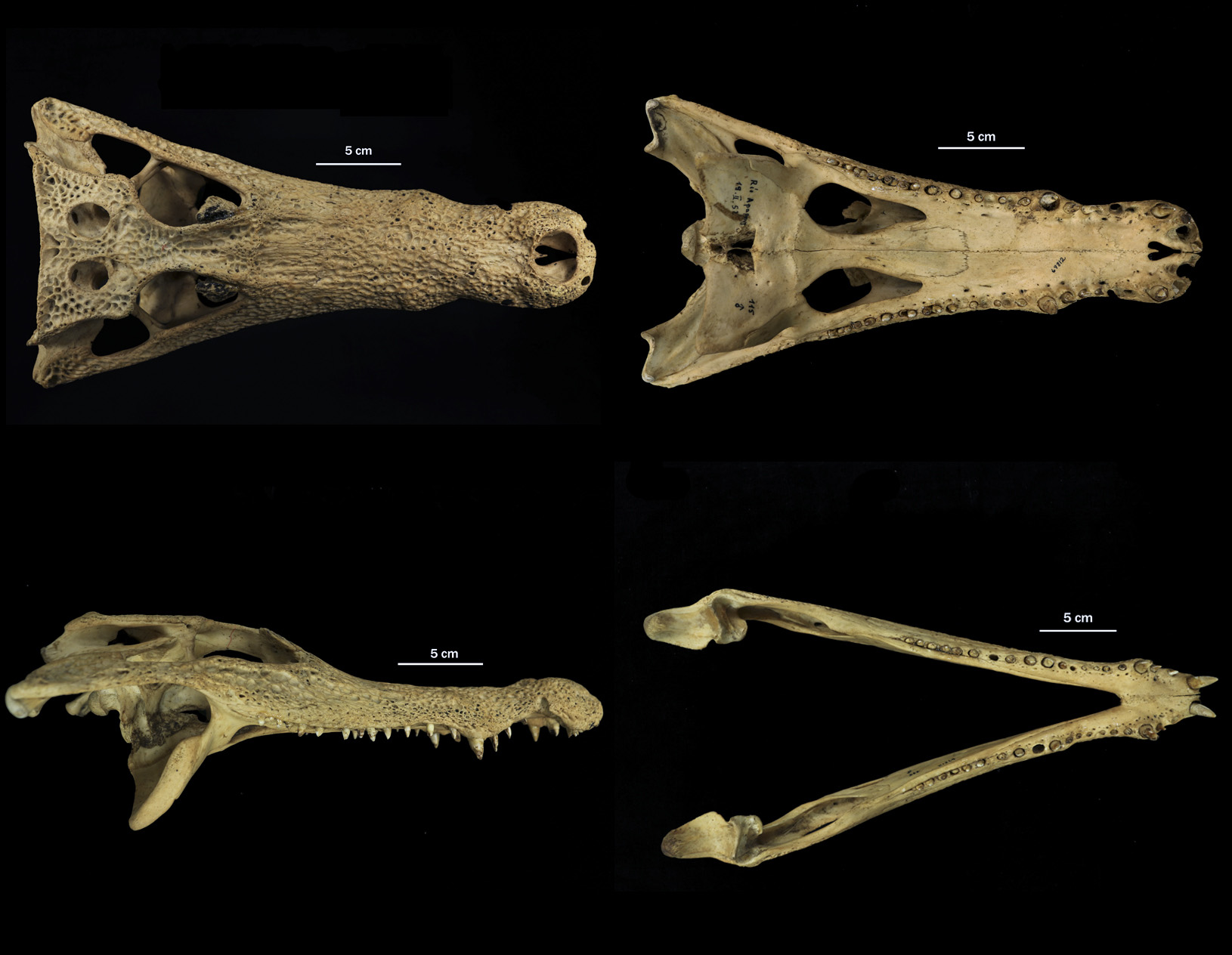
Rio Apaporis Caiman (fig. 1)
Caiman sclerops apaporiensis MEDEM 1955
Caiman crocodilus apaporiensis MEDEM 1955
Caiman crocodilus apaporiensis —NICKEL & AULIYA 2004
Holotype. FMNH 69812, adult male, from upper Rio Apaporis, Amazonas department, Colombia; collected by Federico Medem in February 1952.
Paratypes. FMNH 69813-69832 with the same data as the holotype.
Type locality. Colombia: Laguna Inaná, the upper Apaporis River, Comisariato Amazonas, 0º 7’ N, 71º 0’ W, approximately.
Diagnosis. Differs from other Caiman species by its long skull and large supratemporal fenestrae ( Fig. 1 View FIGURE 1 ). The snout is relatively long and slender in contrast with the rest of extant Caiman species and subspecies. The snout length (anterior tip of snout to anterior orbital border, measured diagonally) is almost twice the width across the anterior orbital border (SL:SW ranged from 1.8 to 2.2). The SL:SW ratio for the other Caiman species/subspecies ranged from 1.2 to 1.7, with C. c. fuscus as broad-snouted taxa (SL:SW ranged from 1.2 to1.4); while C. c. crocodilus showed the most high variation (ranged from 1.4 to 1.7). The premaxillae completely surround the external naris and extend dorsolaterally back to the level of the fifth maxillary alveoli.
Description of the holotype.
Skull: in dorsal view, the skull of C. crocodilus apaporiensis has an elongated form. The snout is very enlarged and narrow in comparison with other Caiman species ( Fig. 1 View FIGURE 1 ; Table 1).
The premaxillae completely surround the external naris and extend dorsolaterally back to the level of the fifth maxillary alveoli. This condition of the premaxillae extending back would be unique condition among alligatorids but shared by basal globidontans (C. Brochu, pers. comm.). In dorsal view each premaxilla extends beyond this point, forming an acute process on either side of the nasal. Each premaxilla supports five alveoli. The second and fourth are largest, and behind the third alveolus their arrangement is almost linear. The premaxillary-maxillary suture on the palate is W-shaped, with each premaxilla bearing a broad posterior process. The nasals are long and narrow in comparison with other caimanine species ( Romer 1956; Bona & Desojo 2011). The nasals broadly contact the premaxillae anteriorly, the maxillae laterally, and lacrimals and prefrontals posterolaterally, and the frontal posteromedially to a small degree. In the anterior part, nasals extend to third maxillary alveolus almost reaching the external naris. In the posterior part, the nasals extend to the level of the posterior end of the maxillae.
The maxillae extend behind the premaxilla and form much of the snout. The maxillae contact the nasals medially and the lacrimals and jugals posteromedially. Contact with the lacrimals is broad. The maxillae and prefrontals are separated by the lacrimal-nasal suture and do not contact each other. The maxillae possess 19–21 alveoli.
The lacrimal forms the anterior margin of the orbit and extends rostrally and modestly contacts the nasal.
The prefrontal forms the anteromedial margin of the orbit. It contacts the lacrimal laterally and the frontal and nasal medially. Posteriorly, between the orbit and frontal, there is a shallow sulcus on the dorsal prefrontal surface.
The frontal is slightly concave between the orbits and flat posteriorly. Anteriorly, it forms an acute process, which contacts the nasals. The frontal contacts the postorbitals posterolaterally and the parietal posteriorly. The posterior margin of the frontal is convex and not involved in the formation of the supratemporal fenestrae.
The postorbital contacts the frontal and parietal in its anteromedial part, and the squamosal posteroventrally. This structure forms the anterior part of the supratemporal fenestrae, the dorsal part of the postorbital bar and pass along the posterior margin of the infratemporal fenestra. The parietal forms the medial walls of the supratemporal fenestrae. The interfenestral bar is dorsally flat. It contacts the squamosals posteromedially. The supratemporal fenestrae are large in comparison with other Caiman species ( Fig. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 ). The parietal perforates on the medial wall of the fenestra. The squamosal contacts the postorbital anteriorly, forming the posterior wall of the supratemporal fenestrae. In ventral view, the maxillae contact the palatines posteromedially and ectopterygoids posteriorly. The maxillae, premaxilla and palatine form the anterior floor of the nasopharyngeal duct. The palatines are slender and contact one another at the midline, delimiting the suborbital fenestrae medially. The palatines exhibit a slight concavity posteriorly. The pterygoids contact each other along the midline conforming the middle and posterior part of the nasopalatine duct. The secondary choana is located in the middle to posterior part of the pterygoids.
The ectopterygoid delimits the suborbital fenestra posterolaterally and contacts the maxilla anterolaterally, the jugal posterolaterally, the pterygoids medially, and the descending process for the postorbital posteromedially.
Jaw: The dentaries meet at the midline along a symphysis that extends back to the level of the fourth or fifth alveolus. The dentary extends back to 14 alveolus. There are 20 dentary alveoli; most are comparatively small, except the first and fourth which is almost twice the diameter of the other alveoli. The pattern of spacing of alveoli in the dentary is regular, except for the more widely spaced first three and closer third and fourth.
The splenials do not participate in the short mandibular symphysis. They are adjacent to the posteriormost five dentary alveoli. The splenial bears a small foramen in the middle, as all alligatorids ( Bona & Desojo 2011). This foramen usually is confused as the foramen intermandibularis oralis, however this foramen is lost in all living alligatorids, excepting Alligator sinensis (C. Brochu, pers. comm.). The splenial makes contact with the coronoid posterordorsally and angular posteroventrally, delimitating the foramen intermandibularis caudalis. The angular lies below the surangular and forms the ventral margin of the external mandibular fenestra. The angular extends more than the internal mandibular fenestra and encapsulates completely the foramen intermandibularis caudalis. The angular contacts the surangular posteriorly, and it extends posterior to the external mandibular fenestra. The surangular projects posteriorly, forming the posterodoral margin of the external mandibular fenestra. The external mandibular fenestra is wide and makes visible laterally the foramen intermandibular caudalis. The surangular exhibits ventral and dorsal process in its anterior part similar in length. The articular forms the posterior margin of the internal mandibular fenestra and its dorsal surface is concave, and bears an anterior process ventral to lingual foramen. The retroarticular process projects posterodorsally. The coronoid is ventrally elongated completely surrounds foramen intermandibularis medius. In its anterior part makes contact with splenial and its posterior end makes contact with angular bone.
Coloration in life (adapted from original description). The color description of living specimens is from Medem (1955): “the dorsal coloration is bright yellowish-brown, with black spots and vermiculations; spotting more dense on the head, making the top of the head dark brown; tail yellowish on light brown, covered with numerous black vermiculations and spots, with four to six broad dark zones discernible; throat and lower jaws yellowish; flanks in general yellow, gray directly beneath the outer rows of dorsal scutes; keeled scutes of the flanks dark brown or black; several scutes with orange-colored keels on the sides of the neck; extremities dark gray or black”.
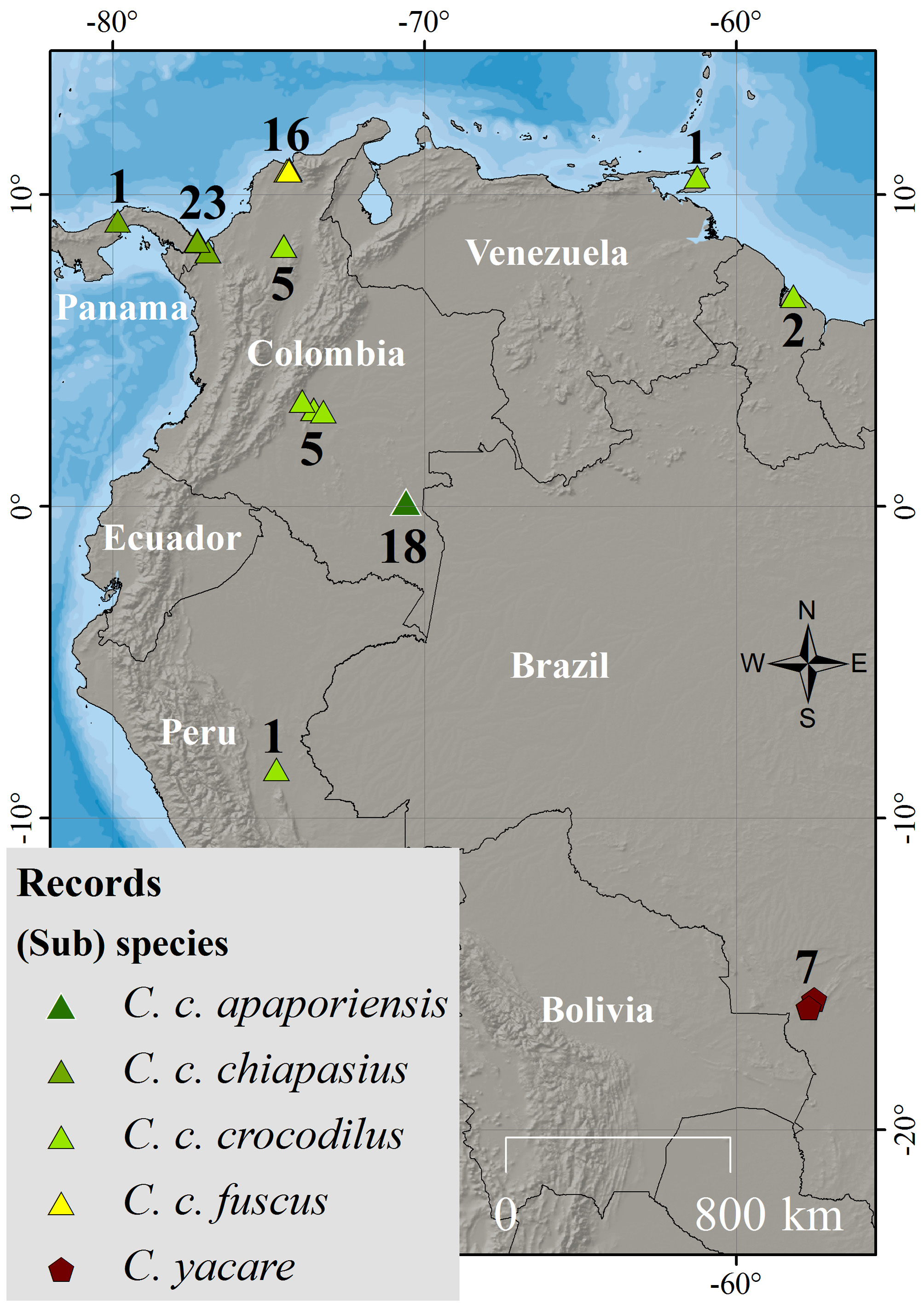
Distribution and ecology. The current distribution of Rio Apaporis Caiman remains uncertain due to the low herpetological sampling conducted in that area of Colombia. We only made reference to historical information from previous studies ( Fig. 6 View FIGURE 6 ). The first surveys conducted by Medem (1955) were around two hundred kilometers between Puerto Yaviya and the Falls of Jirijimo in the upper of Apaporis River. Subsequently, Medem (1981) observed C. c. apaporiensis in the Ajaju and Macayo Rivers. A specimen was collected in the upper Tacunema. Additional observations provided by local landowners who state that the “long-snouted caiman ” have been observed in some lakes of the upper Mesai, tributaries of Yari River in the Caqueta basin ( Fig. 6 View FIGURE 6 ).
TABLE 1. Range of morphological measurements of the holotype and paratypes of C. c. apaporiensis , Caiman yacare and Caiman crocodilus complex.
C. crocodilus apaporiensis C. crocodilus C. crocodilus C. crocodilus C. yacare Conservation status. Assessment of population size, extent of occurrence and occupancy and threats over C. c. apaporiensis are needed to determine the real status of the species, and conservation status definition seems warranted. Speculative and anecdotal information mostly derived from original descriptions of the subspecies by Medem (1955) and our interpretation indicates the sub-species has a likely restricted distribution of no more than 60 km 2, is affected by hunting pressure by local communities ( Medem 1981) and it is likely suffering from genetic introgression and hybridization. Given these factors, it is likely the species could be assessed at high risk, potentially as Critically Endangered according to the IUCN Red List of Threatened Species criteria; however, information needed to adequately provide a Red List category is still lacking. As a conservative approach, we suggest listing the sub-species as Data Deficient, given the enormous information gaps, and prioritize it for further assessment and assigning an adequate conservation status category.
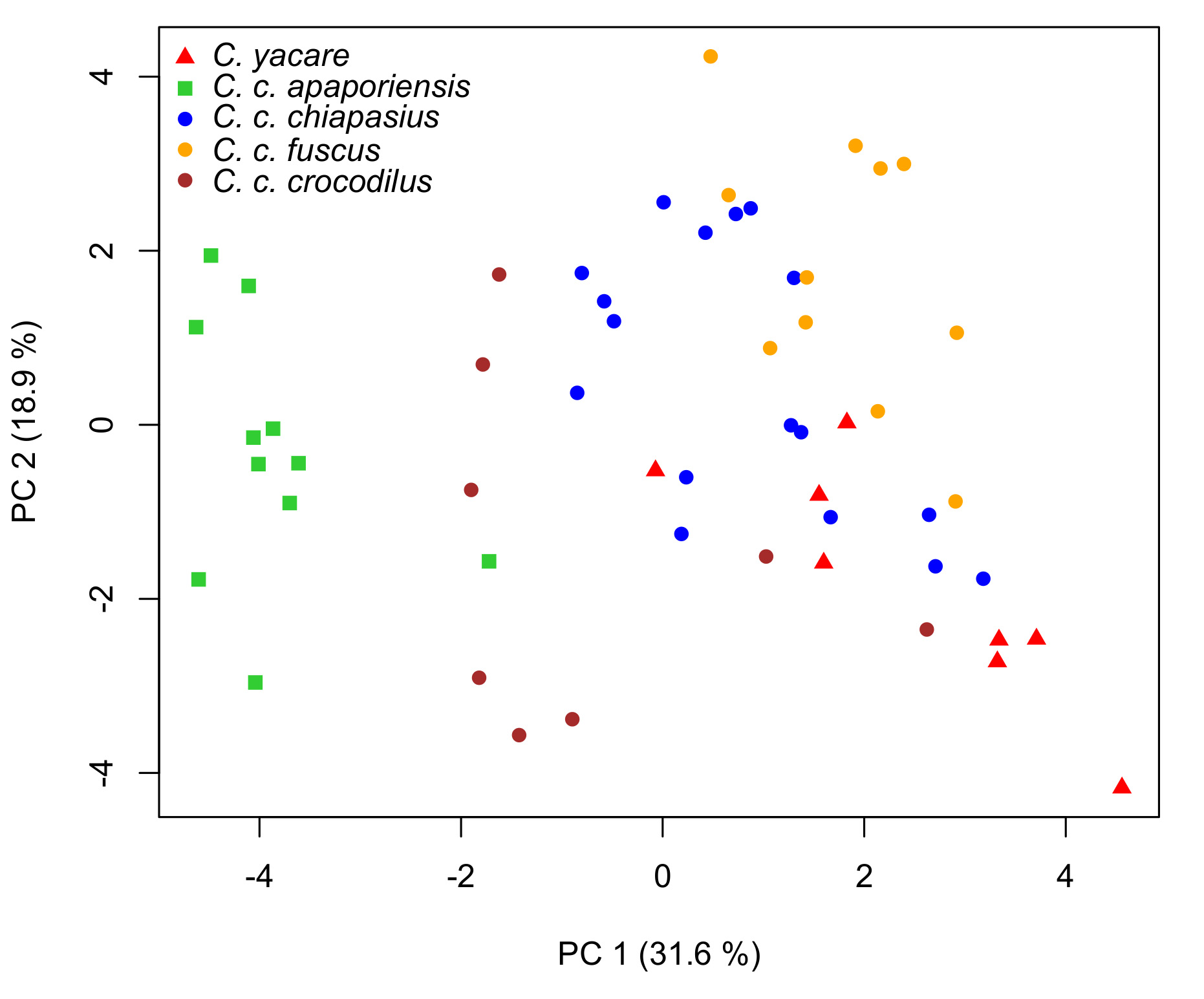
Morphometric analysis. The principal component analysis shows a separation between C. c. apaporiensis , C.yacare , and other C. crocodilus complex forms ( Fig. 7 View FIGURE 7 ). The first four components explain 63.73% of variance in cranial shape ( Table 2). Cranial width variables had the highest loadings in the first component (CW, SW and WMS). Cranial length (DCL, LCR, ML, DL and LM) showed the highest loadings in the second component ( Fig. 7 View FIGURE 7 ); while IOW, OL, VCL, WMS, ML and LMS have the highest loadings in the third and four components. The use of derived discriminant functional analysis (DCL, CW, SL, and SW) showed that between 83.3% and 100% (untransformed and log-transformed data, respectively) was correctly attributed to C. c. apaporiensis . For C. yacare was 87.5% in both cases. For C. c. chiapasius was 29.4% and 52.9%; whereas for C.c. crocodilus and C. c. fuscus was 66.7% and 75.0% in both cases, respectively.
Etymology. The specific name apaporiensis refers to the Apaporis River.
Character PC 1 PC 2 PC 3 PC 4
% variation 31.56% 18.85% 7.49% 5.83%
Eigenvalues 6.63 3.96 1.57 1.22
Loadings DCL -0.0061 -0.3696 0.19676 0.10989 CW 0.33396 0.13360 -0.0133 -0.0572 SW 0.35221 0.14270 -0.04259 0.00694 SL -0.25742 0.22898 -0.26551 0.06294 IOW 0.19604 0.06299 0.31611 -0.53937 OW 0.28979 0.14781 -0.18685 0.08006 OL -0.05417 0.14838 -0.30824 -0.37701 LCR 0.02247 -0.34248 -0.09610 -0.01468 WCR 0.29611 0.09828 -0.16889 -0.02210 WN 0.23989 0.26140 -0.00657 0.05309 VCL -0.01741 0.17818 0.23731 0.32292 UM -0.20773 0.27354 -0.01130 0.07901 PXS 0.19011 -0.00713 -0.31627 0.53329 MXS -0.21932 0.22429 0.03451 -0.21158 ML 0.09016 0.32117 0.32824 -0.01387 LMS 0.17563 -0.16035 0.44366 0.11282 WMS 0.35998 0.07722 0.04460 0.00692 DL -0.12403 0.37020 0.17782 0.14424 DW 0.09452 0.05551 0.25935 0.18471 WSR 0.26283 0.04880 -0.22782 -0.14023 LM -0.19334 0.30884 0.07834 0.10204
| FMNH |
Field Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Caiman crocodilus apaporiensis
| Escobedo-Galván, Armando H., Velasco, Julián A., González-Maya, José F. & Resetar, Alan 2015 |
Caiman crocodilus apaporiensis MEDEM 1955
| Medem 1955 |