Austrjapyx wynbergensis Sendra & Sánchez-García, 2023
|
publication ID |
https://doi.org/ 10.5852/ejt.2023.894.2287 |
|
publication LSID |
lsid:zoobank.org:pub:11C1DFE4-02F2-4FEA-BAD1-ACCAEA3590DB |
|
DOI |
https://doi.org/10.5281/zenodo.10009340 |
|
persistent identifier |
https://treatment.plazi.org/id/A9E9733B-649D-43BE-92CF-F481F010DA8A |
|
taxon LSID |
lsid:zoobank.org:act:A9E9733B-649D-43BE-92CF-F481F010DA8A |
|
treatment provided by |
Plazi |
|
scientific name |
Austrjapyx wynbergensis Sendra & Sánchez-García |
| status |
sp. nov. |
Austrjapyx wynbergensis Sendra & Sánchez-García sp. nov.
urn:lsid:zoobank.org:act:A9E9733B-649D-43BE-92CF-F481F010DA8A
Figs 1‒7 View Fig View Fig View Fig View Fig View Fig View Fig View Fig
Etymology
The specific epithet “wynbergensis” refers to the cave inhabited by the species.
Type material
Holotype SOUTH AFRICA • ♀; Cape Town, Wynberg Cave ; 33°59′10.48″ S, 18°24′10.64″ E; 12 Oct. 2019; Rodrigo Lopes Ferreira leg.; labelled “♀1-holotype SAM-MNW-CO15126 ”; ISAM. GoogleMaps
Paratypes
SOUTH AFRICA • 1 ♀; same collection data as for holotype; labelled “♀2-paratype-MZB ( MCNB) 2023-0618”; MZB GoogleMaps .
Other studied material
SOUTH AFRICA • 1 ♀; same collection data as for holotype GoogleMaps ; AS.
Description
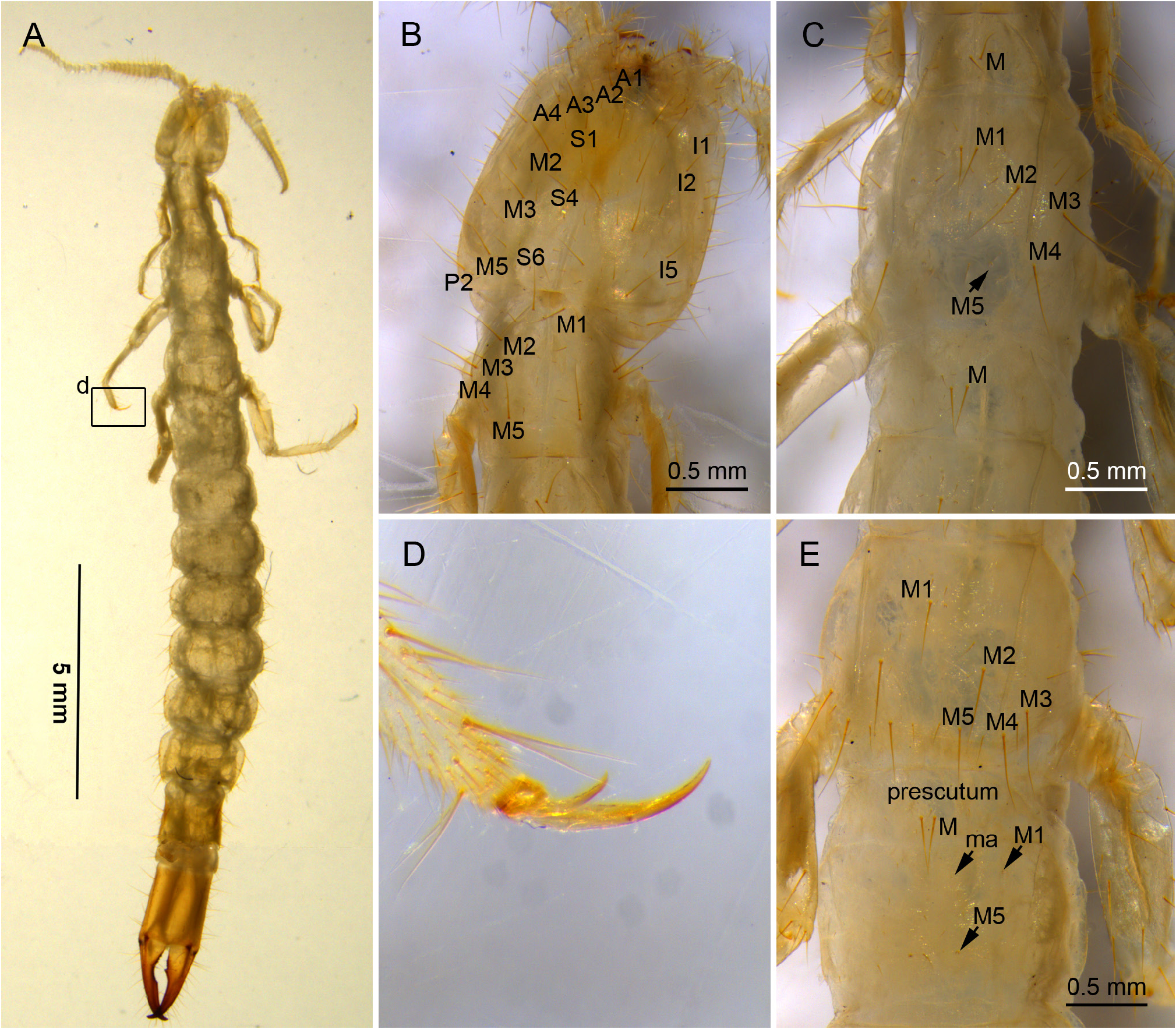
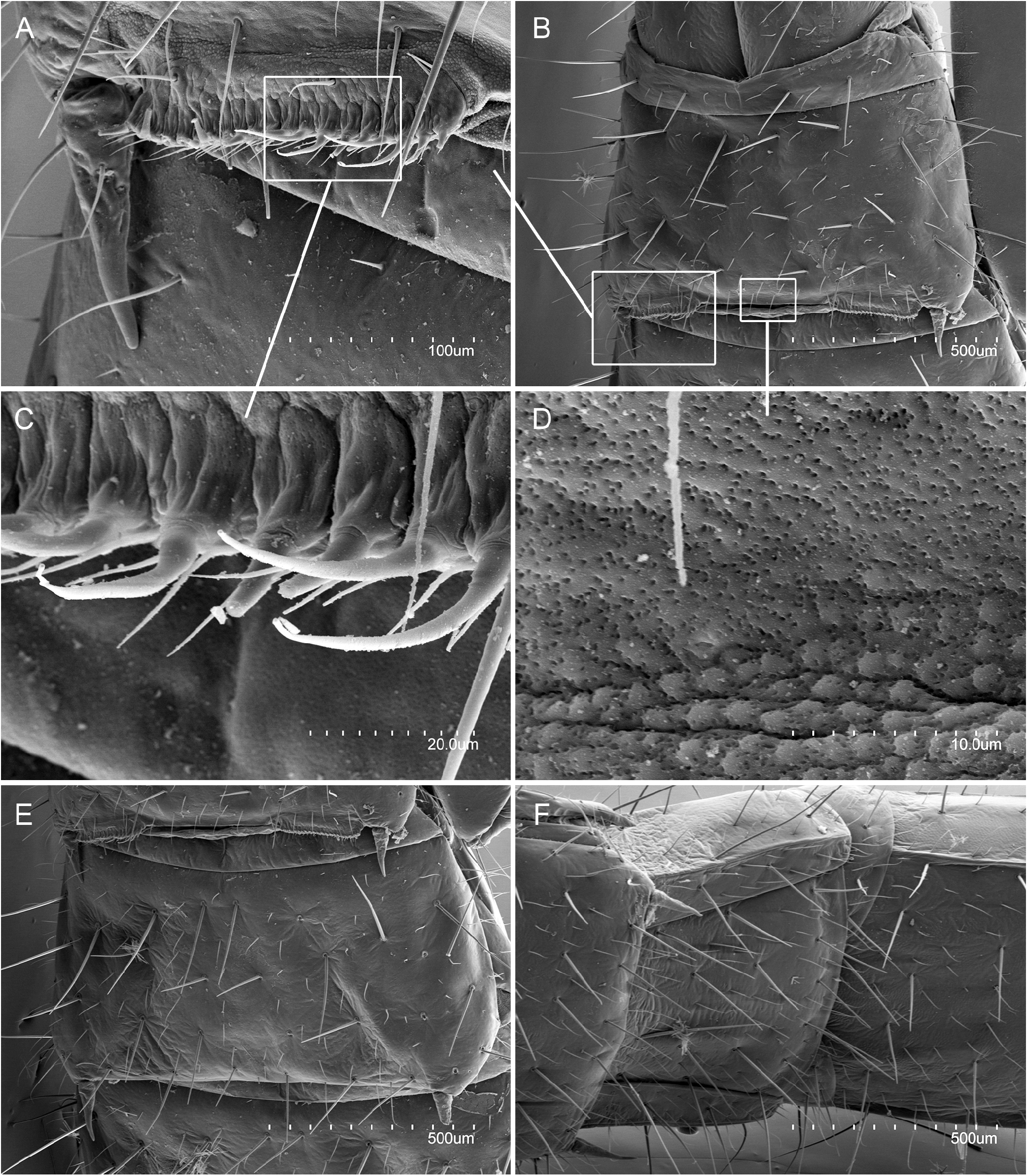
BODY. Elongate ( Fig. 1A View Fig ), length 15 mm in ♀ 1- holotype, 8.8 mm in ♀ 2- paratype. Maximum width at urotergite VII 1.7 mm in ♀ 1- holotype, 1.1 mm in ♀ 2- paratype. Epicuticle smooth under optical microscope and with numerous micropores at higher magnifications (sternites with 4‒5 micropores/μm 2, diameter 0.2‒0.3 μm; urite X with 3‒4 micropores/μm 2, diameter 0.10‒0.15 μm) ( Fig. 5D View Fig ). Body with s, sM, and M setae, and with few or no ms. Cuticle unpigmented, with sclerotized areas on mandible tips, femoral and tibial condyles, abdominal segments VIII‒X and cerci.
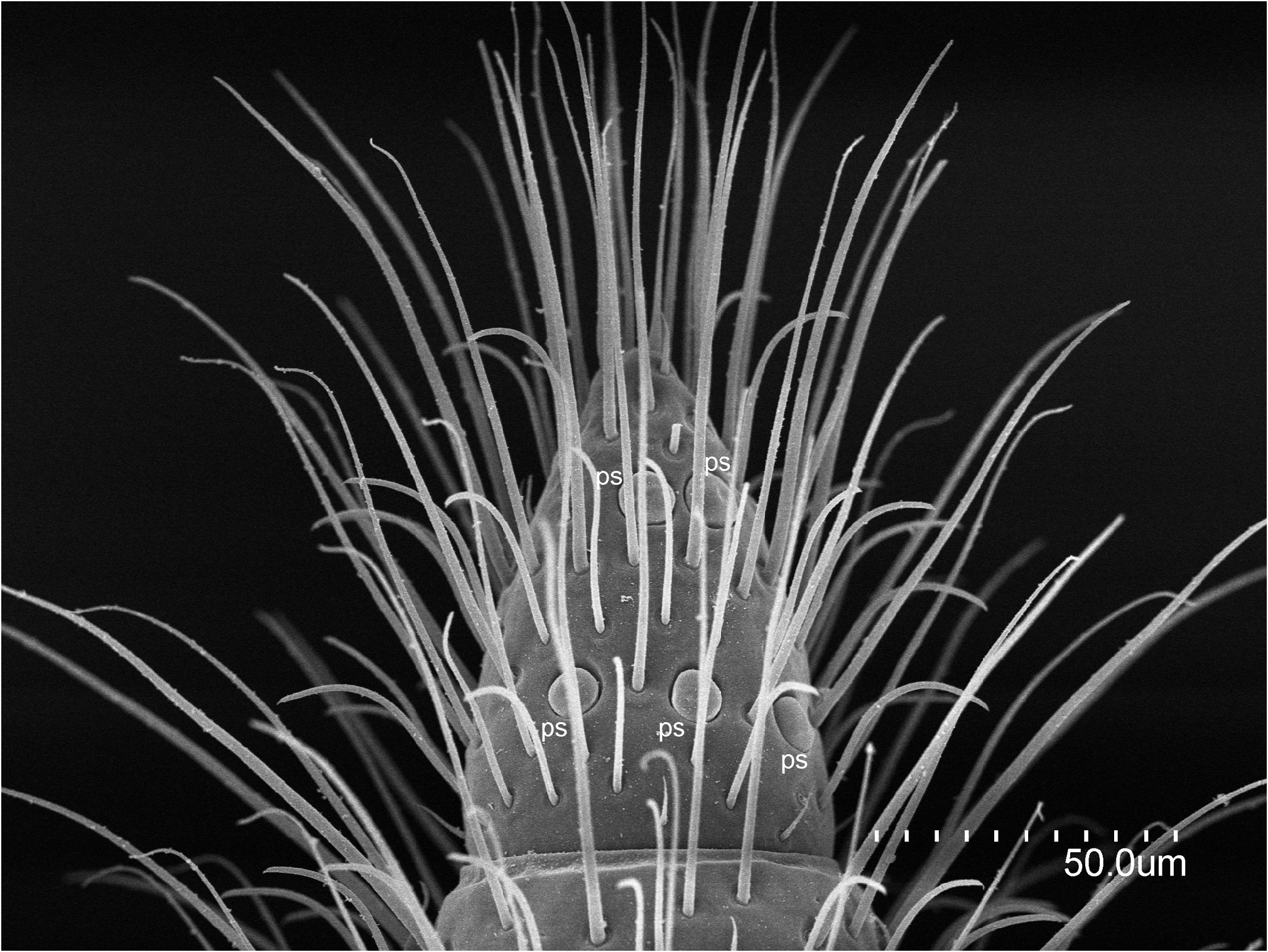
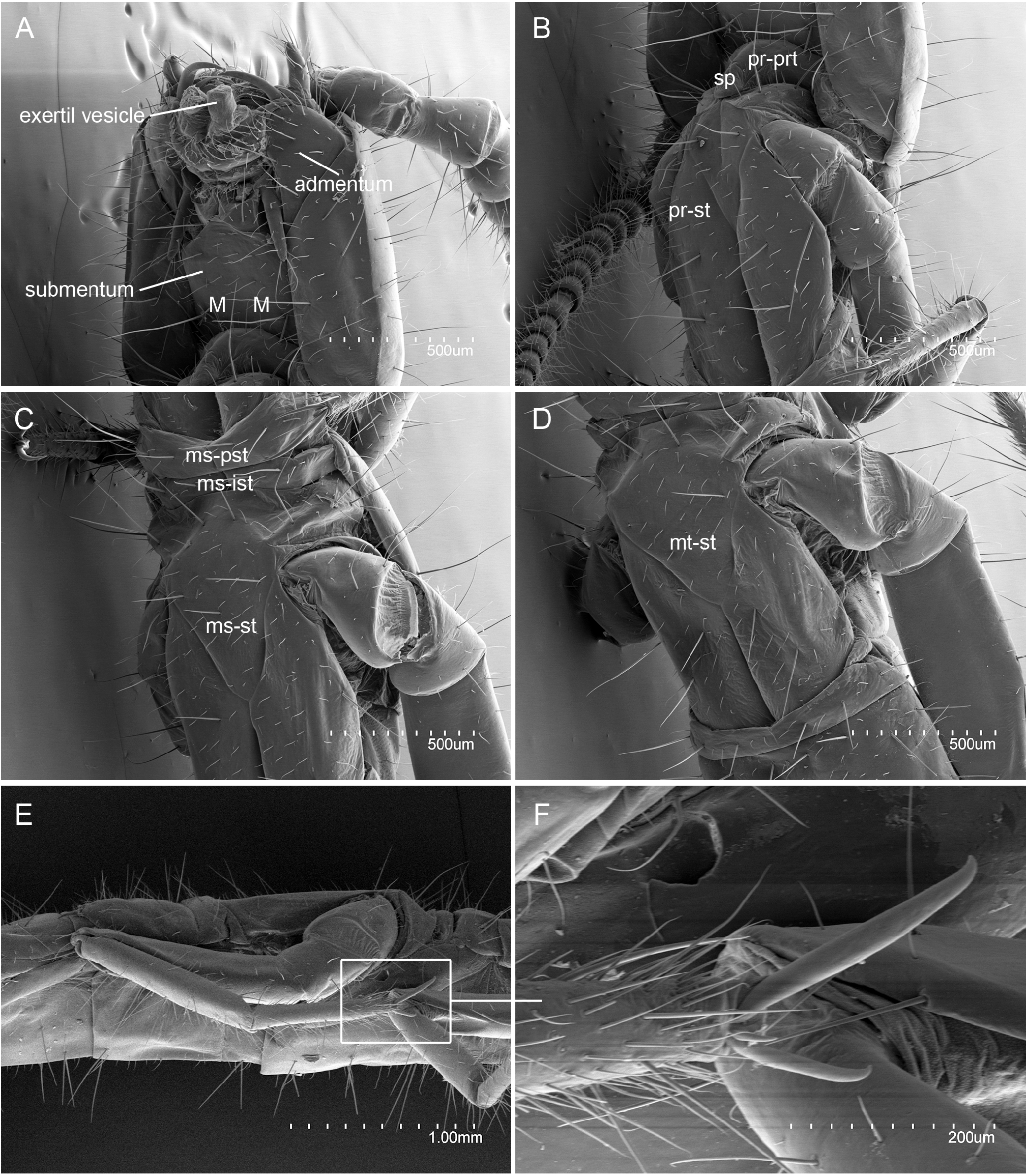
HEAD. Antenna length 3.5 mm, 0.4× as long as body, with 31 antennomeres; antennomeres telescopate in holotype, basal antennomere short, followed by slightly longer antennomeres II and III, 1.3× as long as wide; medial antennomeres as long as wide. All antennomeres with sM, a few ms, and abundant s setae; sM apparently distributed in two whorls. Trichobothria present on antennomeres IV‒VI in a 2/3/3 pattern, with a trichobothria in distal position. Apical antennomere with 10 placoid sensilla distributed in two irregular whorls ( Fig. 2 View Fig ). Dorsal and ventral side of head with a few sM and several M setae; on dorsal side: A1‒4, S1, 4, 6, M2‒3, 5, I1‒2, I5, L1‒3 and P2 macrosetae ( Fig. 1B View Fig ); on ventral side: submentum with large 1+1 M in posterior position, admentum with 9+9 M, mentum at base of labial palps with 1+1 M; external lobes of mentum with abundant sM; the pair of exertil vesicles of the external lobes visible in the holotype. Labial palp elongate, 9× as long as wide, with one proximal sM and five medial and distal sM. Lacinia falciform, well sclerotized, all five laminae pectinate ( Fig. 3A View Fig ).
THORAX. Thoracic segments elongate. Pronotum with 5+5 M1‒5 ( Fig. 1B View Fig ); prescutum of mesonotum with 1+1 M; mesonotum with 5+5 M1‒5 and a few sM ( Fig. 1C View Fig ); prescutum of metanotum with 1+1 M, metanotum with 5+5 M1‒5 and a few sM ( Fig. 1E View Fig ). Thoracic sternites, intersternites, and presternites well-defined, with a few s and M setae. Pro-presternites and pro-, meso- and metasternites with strong internal Y-shaped cuticular structures (furcisternites) ( Barlet & Carpentier 1962); only in pro-presternites the prolongation of the posterior branch (spine) is visible on the surface ( Denis 1949). Pro-presternum with 1+1 lateral anterior M; prosternum with 1+1 medial anterior M, 3+3 lateral anterior M, 1+1 medial posterior M, and 2+2 lateral posterior M; meso-poststernum with 3+3 M; meso-intersternum with 3+3 M; mesosternum with 1+1 medial anterior M, 2+2 lateral anterior M, 1+1 medial intermediate M, 1 sagittal M, 1+1 medial posterior M and 2+2 lateral posterior M; meta-poststernum with 4+4 M; meta-intersternum with 3+3 M; and metasternum with 1+1 medial anterior M, 3+3 lateral anterior M, 1+1 medial intermediate M, 1+1 sagittal M, 1+1 medial posterior and 2+2 lateral posterior M ( Fig. 3D View Fig ). Legs elongate, hind leg 3.6 mm long, reaching sixth abdominal segment in holotype. Femur-tibia-tarsus articulations with a row of sM setae; coxa with 1 ventral M; trochanter with 3 ventral M; femur with 8 ventral and 5 dorsal M; tibia with 4 ventral and 5 dorsal M; tarsus with 5 dorsal M and abundant sM plus two ventral rows of 4 long, thick setae. Pretarsus with two rather unequal claws and a sharp medial unguiculus; posterior claw 1.7× as long as anterior claw ( Fig. 3E‒F View Fig ).
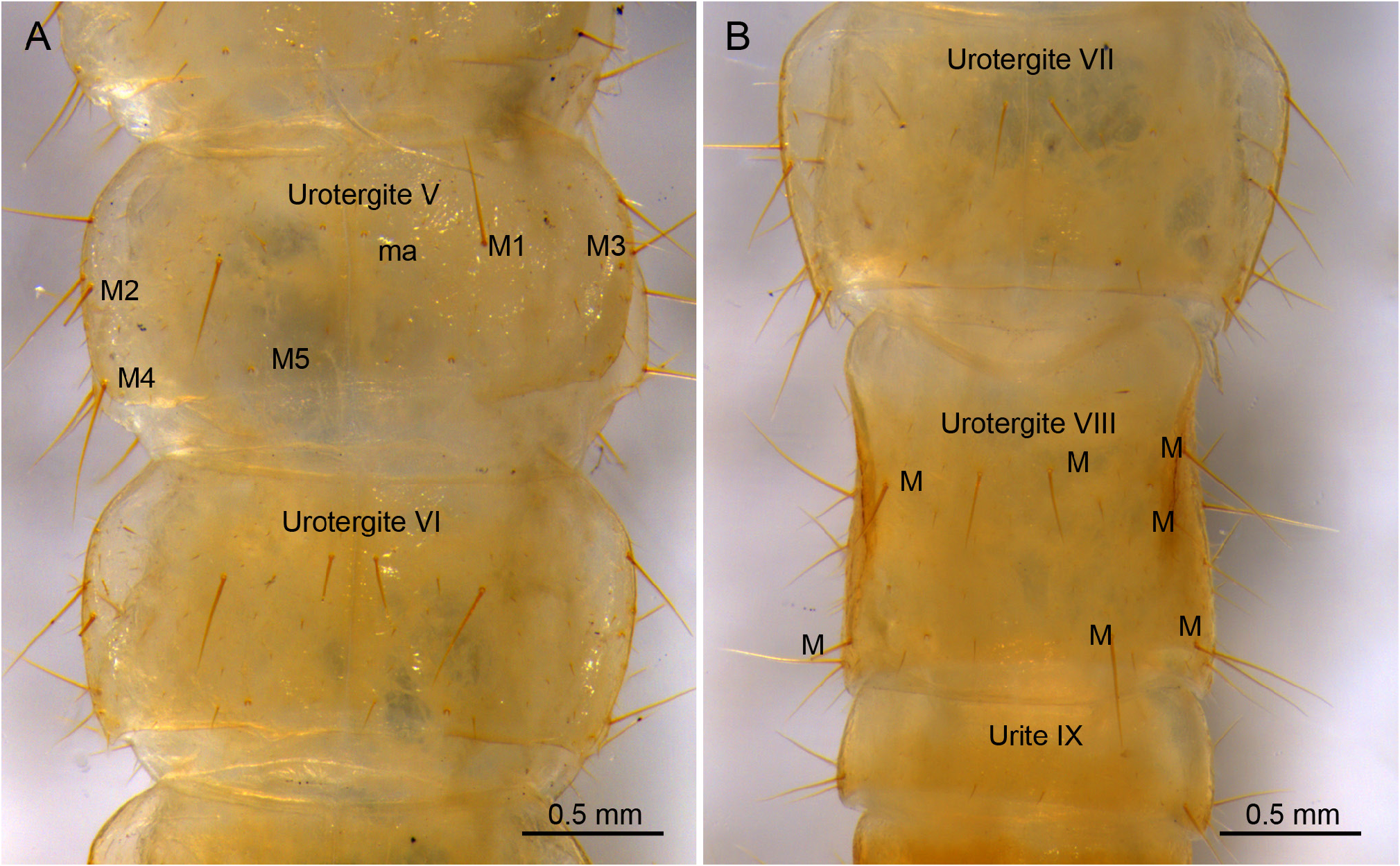
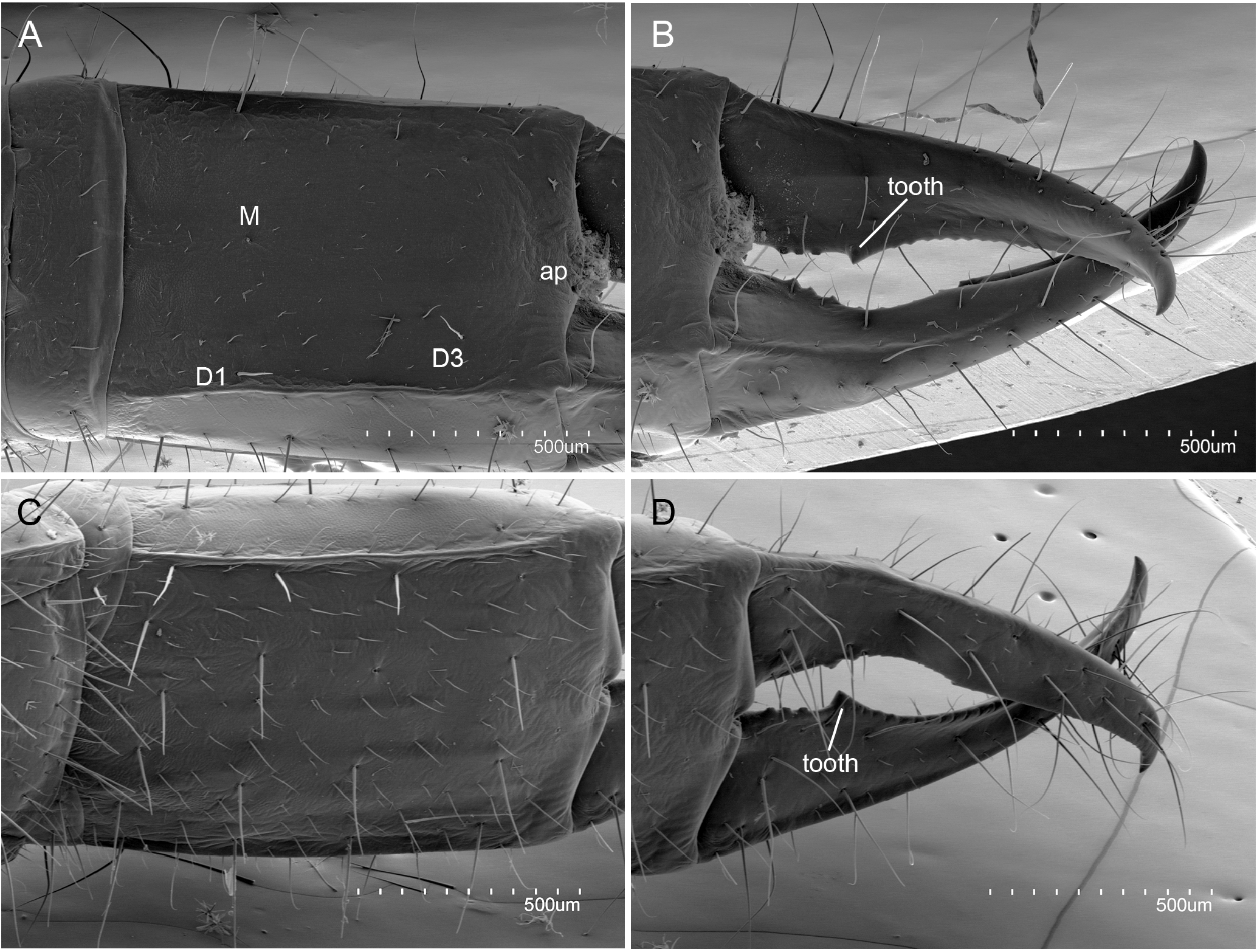
ABDOMEN. Abdominal tergites with large M, and a few sM and ms. Prescutum of urotergite I with 1+1 M, scutum with 1+1 M (ma), 1+1 M1 and 1+1 M5 ( Fig. 1E View Fig ); urotergites II‒VII with 1+1 M (ma) and 5+5 M1‒5 ( Fig. 4A View Fig ); urotergite VIII with 7+7 M ( Fig. 4B View Fig ); urite IX with 5+ 5 M. Urite X 1.6× as long as wide, with distinctly marked carinae; carinae with subparallel margins slightly converging towards anterior border; dorsal side with 2+2 M intracarinal D1, 3 (plus one sagittal M between D1), acropygium rounded ( Fig. 6A View Fig ); lateral side with 3+3 M (L1, 3, 5); ventral side with four rows of 3+3 M, 3+3 M, 2+2 M and 2+2 M ( Fig. 6C View Fig ). Urotergites I‒VI with blunt, slightly rounded posterolateral angles; angles in tergites VII and VIII with sclerotized tip; rounded and sclerotized in tergite IX ( Fig. 4 View Fig ). Urosternites with s and strong M. Surface of urosternite I multiperforated and with rounded protrusions ( Fig. 5D View Fig ); prescutum with 3+3 M; scutum with 12+ 12 M. Median glandular organ without setae or disculis. Lateral subcoxal organ with one row of glandular setae (GS) (19 GS in holotype; 18 GS in ♀ 3- paratype and 6 GS in ♀ 2- paratype) and one row of sensory setae (SS) (about 40 SS in holotype and ♀ 3- paratype); lateral subcoxal organ occupying 0.2‒0.3 of interstylar area; GS/st1 (stylus of first sternite) = 0.3, SS/st1= 0.15 ( Fig. 5A–B, E View Fig ). Urosternites II‒VII with 7+7 A M, 4+4 B M, and 5+5 C M; urosternite VIII with 3+3 A M, 2+2 B M, and 3+3 C M ( Fig. 5F View Fig ). Cerci asymmetric, strong, well-developed, elongate, straight in the proximal half and curved in the distal half, ending in a hook ( Fig. 6B, D View Fig ); length ranging from 1.32 mm in holotype to 0.6 mm in ♀ 2- paratype, always slightly shorter than urite X; heavily sclerotized, with dorsal and ventral outer carinae arising from dorsal and ventral acetabular articulations; carinae extending almost to apex ventrally and halfway dorsally. Cerci dorsally concave and with the distal end slightly upward. Right cercus with postmedial tooth pointed; predental margin with a row of three round denticles; postdental margin with a row of more than 20 small denticles gradually decreasing in size until disappearing near the hook. Left cercus with two rows of denticles: superior predental row with three proximal denticles followed by 4‒6 small, round denticles; inferior predental row with 12 round denticles starting with a large proximal one and ending in a very small one; the inferior row prolonged in a postdental, knife-shaped margin up to the hook, with a few tiny denticles distally. Campaniform sensilla distributed in the distal part of the cerci.
Taxonomic affinities
Austrjapyx wynbergensis sp. nov. with its premedial tooth in the right cercus and its denticles pattern, two rows in the left cercus and a single row on the right, led us to cautiously include it in the genus Austrjapyx following Pagés (1952) criteria. Nevertheless, a protruding knife-shaped distal margin on the left cercus of Austrjapyx wynbergensis separates it from the two African species of Austrjapyx : Austrjapyx leleupi Pagés, 1952 and Japyx proditus Silvestri, 1918 , considered by Pagés (1952) as Austrjapyx proditus but with no formal taxonomical designation. The peculiar knife-shaped distal margin on the left cercus is not seen in the 13 species of Austrjapyx already described from South America where the genus is more diversified ( Silvestri 1948a; Smith & Gonzalez 1964). Autrjapyx leleupi seems the closest species to A. wynbergensis sp. nov. due to seven geographical, ecological, and morphological reasons: (1) geographical proximity, both are from Africa and A. leleupi was collected near Mbanza-Ngungu, Democratic Republic of Congo (Central Province in the west), about three thousand kilometres to the north of the type locality of A. wynbergensis ; (2) both inhabit cave ecosystems; (3) they exhibit an elongated body and appendages and have more than ten large and protruding placoid sensilla; (4) they share the same pattern of thorax macrosetae (M); (5) there are similarities in the shape and distribution of macrosetae (M) in the abdomen; (6) their first urosternal organs, both medial and lateral, are similar; and (7) both have roundish lateral angle urotergites. Despite the similarities, A. wynbergensis differs from A. leleupi in at least five morphological features: (1) the knife-shaped distal on the left cercus’ margin; (2) 30 antennomeres in A. leleupi instead of 31 in A. wynbergensis ; (3) scutum of urotergite I with 3+3 macrosetae (M) in A. wynbergensis , that are absent in A. leleupi ; (4) no small setae or pseudospores visible in medial organ in A. wynbergensis , but a dozen in A. leleupi ; and (5) 2+2 macrosetae (M) on dorsal side of utite X in A. wynbergensis , instead of 4+4 M in A. leleupi .
Habitat
The Wynberg Cave, along with other caves (such as Bats, Climbers, Giants, Hangman, Metro, and Smugglers caves), forms the largest cave system in the Cape Peninsula region (with more than 1.2 km in length), which is referred to as the Wynberg Cave System (WCS; Ferreira et al. 2020) ( Fig. 7 View Fig ). The caves are associated with quartzite rocks, occurring at altitudes ranging from 450 m to 750 m a.s.l. The region presents a temperate climate, with hot and dry summers and cold and humid winters ( Marker & Swart 1995). The average temperature inside the caves is around 10°C ( Sharratt 1998). Most caves within the Table Mountain National Park are located at relatively high altitudes, presenting predominantly vertical passages of different sizes. The WCS presents a remarkable cave-restricted fauna, especially if we consider the endemicity and rarity of some species. Ferreira et al. (2020) listed 19 cave-restricted species occurring in the WCS and is one step closer to becoming a subterranean biodiversity hotspot ( Ferreira et al. 2020).
Sharratt (1998) mentioned the presence of a troglobitic Dermaptera DeGeer, 1773 in the WCS. It is possible that the authors misidentified the specimens of Austrjapyx wynbergensis sp. nov. given the morphological similarities with eyeless earwigs ( Ferreira et al. 2020). Hence, the new species herein described may have been known for the last two decades.
The two specimens of Austrjapyx wynbergensis sp. nov. in this study were found under fallen rocky blocks in the lower level of the cave. This species is quite rare since Sharratt (1998) registered only a few specimens found in the Wynberg and Bats caves in deep cave zones (assuming that this species corresponds to the troglobitic Dermaptera mentioned by that author). Unfortunately, there are no data regarding any biological aspects for this new species despite having been registered on the WCS for a long time.
The main threat to cave species in the Table Mountain area is human visitation ( Sharratt 1998; Ferreira et al. 2020). The uncontrolled recreational use of these caves crushes the fauna and alters the microhabitats.Additionally, such activities can pollute terrestrial and aquatic environments with waste (including batteries containing toxic chemicals). Furthermore, these visits can disturb the bat population and alter the cave’s temperature and moisture conditions. Ferreira et al. (2020) verified several impacts in the cave, such as graffiti on the walls and passages with intense trampling. We need to implement emergency measures to ensure the conservation of this cave from the uncontrolled human impact ( Ferreira et al. 2020).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Japygoidea |
|
Family |
|
|
Genus |