Aphis ( Aphis ) rogerblackmani Nieto Nafría, Ortego & Mier Durante, 2022
|
publication ID |
https://doi.org/10.11646/zootaxa.5183.1.31 |
|
publication LSID |
lsid:zoobank.org:pub:15F12672-AC19-49B5-A3D7-6B13359AF400 |
|
DOI |
https://doi.org/10.5281/zenodo.7075630 |
|
persistent identifier |
https://treatment.plazi.org/id/03FF8784-FFD2-FFCB-E8FC-FB5BFB22FAB2 |
|
treatment provided by |
Plazi |
|
scientific name |
Aphis ( Aphis ) rogerblackmani Nieto Nafría, Ortego & Mier Durante |
| status |
sp. nov. |
Aphis ( Aphis) rogerblackmani Nieto Nafría, Ortego & Mier Durante sp. n.
Types. Holotype: apterous viviparous female ARG-1833- 1 (mounted with a paratype): ARGENTINA: RÍO NEGRO: Bariloche: San Carlos de Bariloche ( 41º 08' S 71º 14' W, 870 m), on an unidentified Baccharis species, 18-April- 2012, collection of the Universidad de León GoogleMaps . Paratypes: 4 apterous viviparous females, 1 alate viviparous female, 12 oviparous females and 8 males, same data as the holotype, collection of the Universidad de León GoogleMaps .
Etymology. The specific epithet of Aphis rogerblackmani sp. n. is dedicated to our colleague and friend, the late Roger L. Blackman, for his outstanding work on aphid taxonomy and in memory of his first entomological works, which were developed in the Argentine Patagonia.
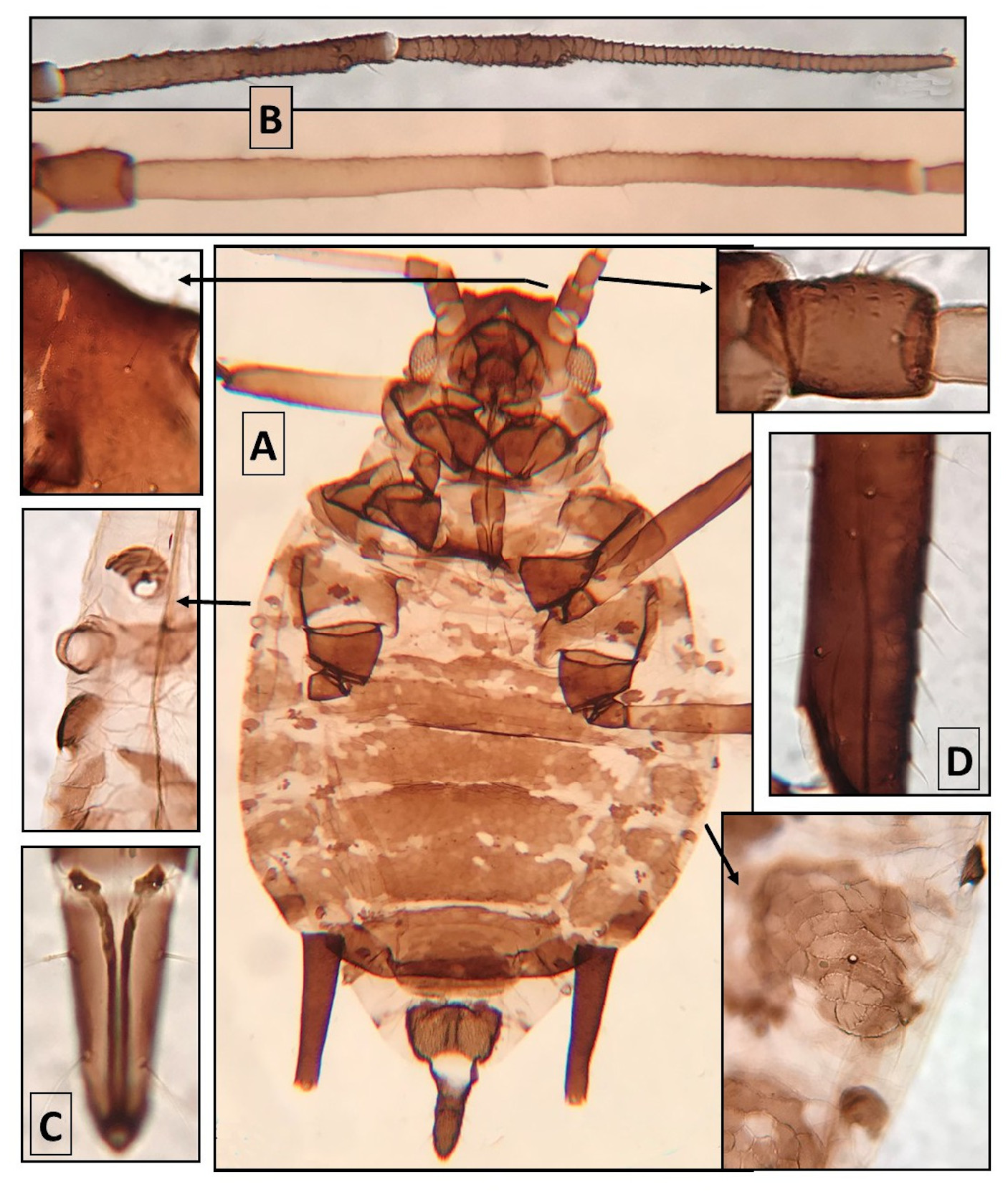
Apterous viviparous females ( Fig. 1 View FIGURE 1 ). From 5 specimens. When alive black. In mounted specimens, head dark brown, almost dorsally smooth, without reticulation, and ventrally striated. Frons wavy. ANT.I–II also almost smooth. ANT.III with 9–12 ST. URS thick and with straight edges. TH widely sclerotized and pigmented. ABD.1–6 with wide spino-pleural bands and MG patches, in some segments contacting each other. ABD.7–8 with wide transverse bands. Intersegmental sclerites conspicuous, as dark as the segmental and spiracular sclerites. PRI MG TUB relatively small, especially those on ABD.7. COM MG TUB, usually absent ( two specimens have one). ST long, slender, pointed, and pale. Coxae and trochanters dark. Tarsal formula, 3.3.2 or 3.3.3. SIPH long and tapering to apex, darker than the TH and ABD sclerotized parts. ABD.8 with 2 ST. Genital plate with 2–5 discal and 8– 12 posterior ST. Cauda lanceolate, with small midway constriction; with 7–11 ST. Other qualitative features in “Common features of the new species”. Metric features in Table 2 View TABLE 2 .
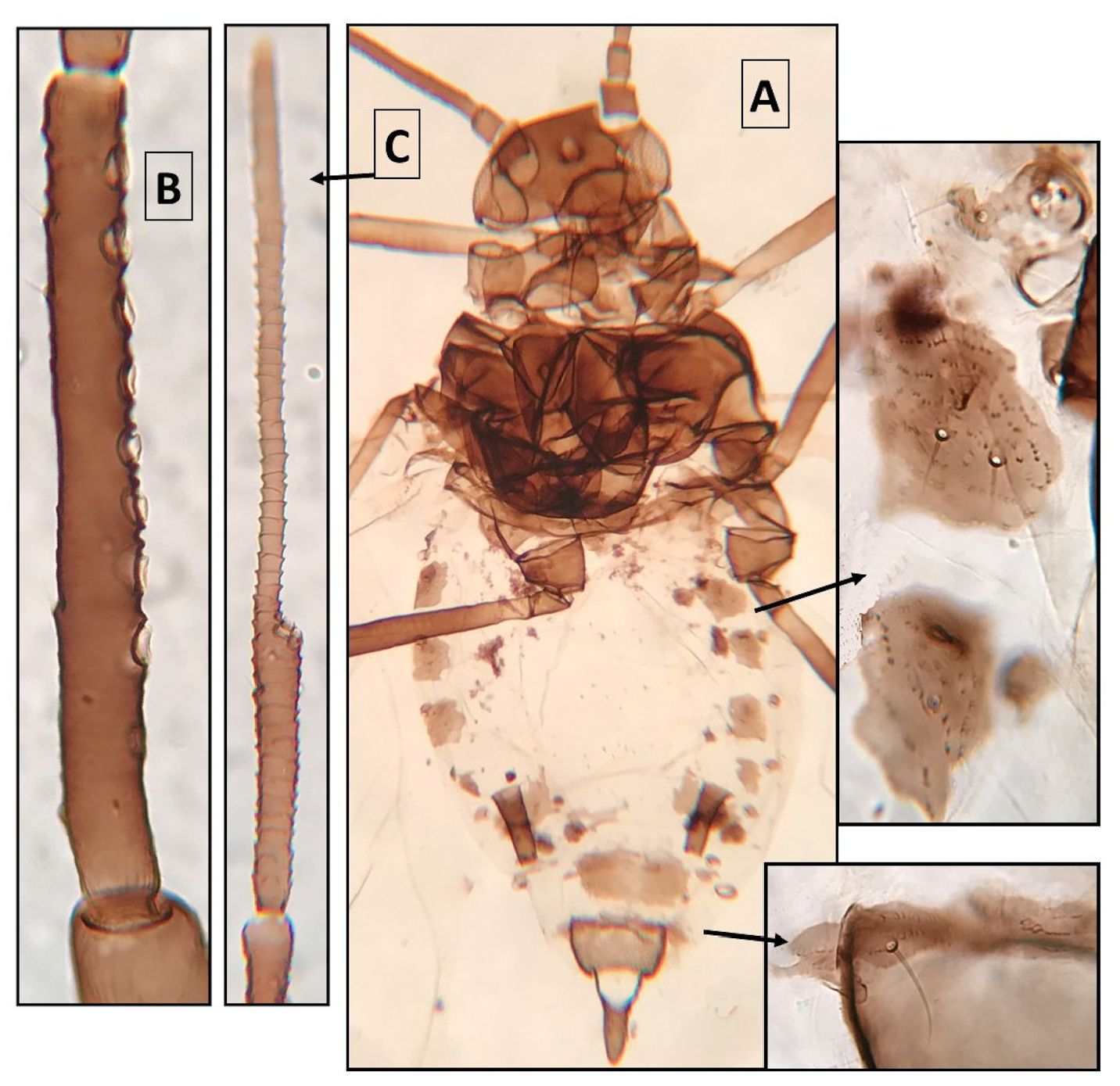
Alate viviparous females ( Fig. 2 View FIGURE 2 ). From 1 specimen. ABD.2–5 mostly membranous, with MG patches; ABD.6 with small spinal sclerites and wide MG patches; ABD.7–8 with thin transverse bands. ANT.III with 10–14 SEC SEN. ANT.IV with 5–6 big and poorly aligned SEC SEN. Other qualitative features in “Common features of the new species”. Metric features in Table 2 View TABLE 2 .
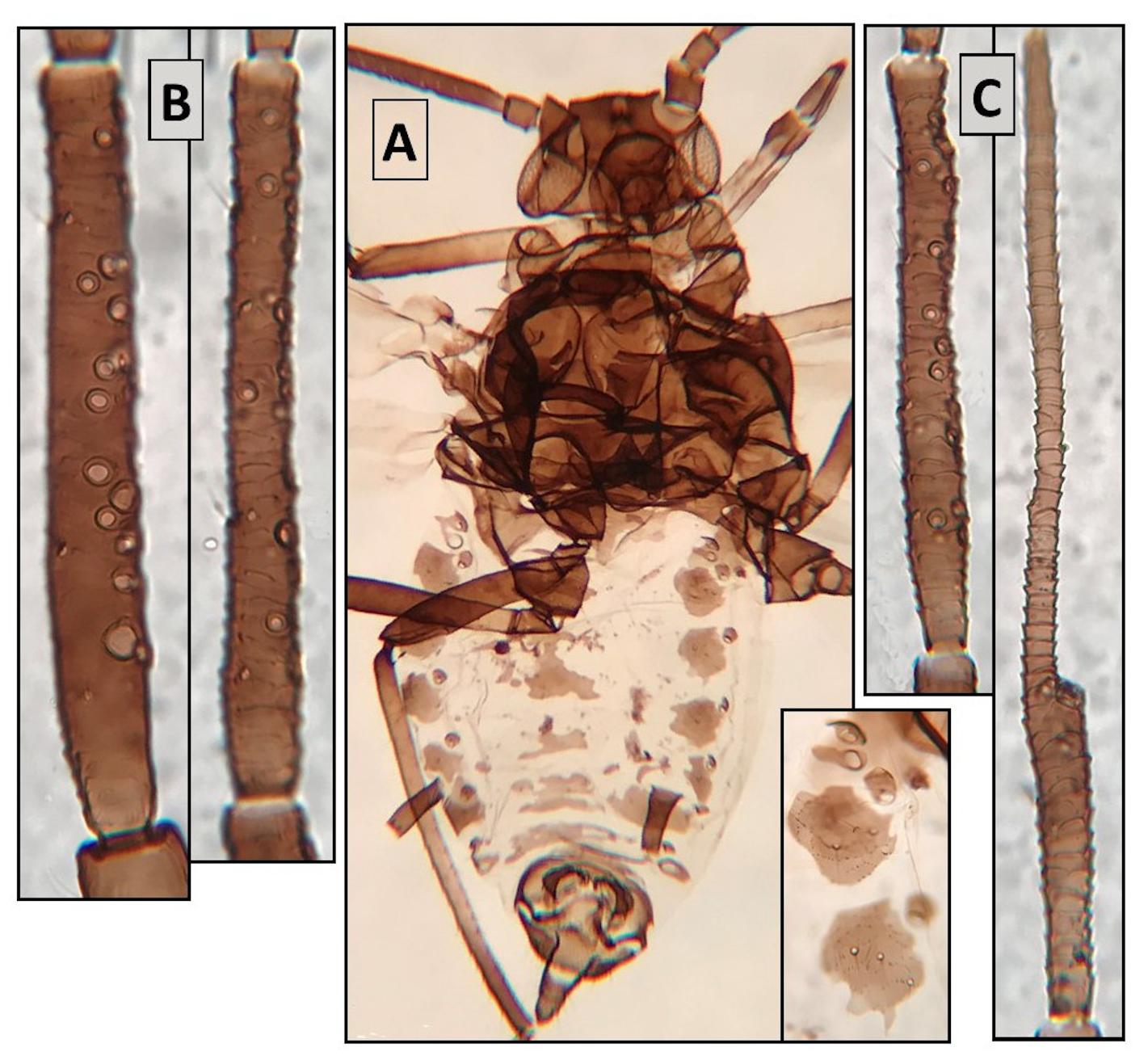
Oviparous females ( Fig. 3 View FIGURE 3 ). From 12 specimens. Like apterous viviparae, with the typical features of this morph. TH and ABD sclerotization and pigmentation diverse, from like those in apterous viviparae to a complete absence. Hind tibiae not very thickened and carrying (48)70–126 scent plates, often twins. ABD.8 and cauda with 5–9 and 8–12 ST respectively. Genital plate with 30–42 ST. Metric features in Table 2 View TABLE 2 .
Males ( Fig. 4 View FIGURE 4 ). From 8 specimens. Winged and very similar to alate viviparae, with: (1) ANT longer, very dark, and with more SEC SEN: 23–32 on ANT.III, 13–22 on ANT.IV and 11–19 on ANT.V; (2) ABD.2–6 with transverse spinal or spino-pleural bands in addition to MG patches; (3) darker legs; (4) shorter and thinner SIPH; and (5) triangular cauda. Parameres conical, robust, and dark brown. Metric features in Table 2 View TABLE 2 .
Bionomics and distribution. Aphis rogerblackmani sp. n. is holocyclic and presumably monoecious on species of Baccharis and perhaps on species of close genera.
It is only known in one locality, whose environmental characteristics are the same to those existing in a large part of western Argentine provinces of Mendoza, Neuquén, Río Negro and Chubut.
......Continued on the next page
Taxonomic discussion, diagnosis. TH and ABD sclerotization, lengths of ANT.VI.PT and ST allows the identification of the new species. A. rogerblackmani sp. n. pairs with A. marthae Essig, 1953 in the first proposition of couple I of the key to apterous viviparae of Aphidina species known in South America by Nieto Nafría et al. (2019). Viviparae of both species can be separated from each other by ABD.2–5 sclerotization: apterae and alatae of A. marthae respectively have discal plate and spino-pleural bands, while those of A. rogerblackmani sp. n. respectively have bands and at most scattered sclerites; in addition, the cauda is triangular in viviparae of A. marthae while it is lanceolate in A. rogerblackmani . The host plant of A. marthae is Quillaja saponaria (Quillajaceae) .
The viviparous females of Aphis rogerblackmani sp. n. could be confused with those of A. ingeborgae , which also lives on Baccharis , for a similar ABD sclerotization and pigmentation, but the peculiar shape of the MG TUB of A. ingeborgae and the length of ANT.VI.B, ANT.VI.PT, HT.2, cauda and ST allow the separation of both species (see Nieto Nafría, 2019: Fig. 1 View FIGURE 1 , page 157, and Table 1 View TABLE 1 , pages 160-161 for A. ingeborgae ).
The differences of apterous viviparae of A. rogerblackmani from those of the other species of Aphis that are known in southern South America living on Asteraceae are presented below in an identification key.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.