Anthothela grandiflora ( Sars, 1856 )
|
publication ID |
https://doi.org/10.11646/zootaxa.4304.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:3D557C94-0783-4C39-80C3-9C321DA94800 |
|
DOI |
https://doi.org/10.5281/zenodo.6015361 |
|
persistent identifier |
https://treatment.plazi.org/id/039B87ED-3E52-FF91-FF4B-E4BF7FD6DEDD |
|
treatment provided by |
Plazi |
|
scientific name |
Anthothela grandiflora ( Sars, 1856 ) |
| status |
|
Anthothela grandiflora ( Sars, 1856) View in CoL
( Figs. 4–15 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 View FIGURE 15 )
Briareum grandiflorum Sars, 1856: 63 –65, Pl. X Figs. 10–12 View FIGURE 10 View FIGURE 11 View FIGURE 12 ; 1857: 238; Storm 1879b: 123; 1892: XXVII.
Anthothela insignis Verrill, 1879a: 15 View in CoL .
Anthothela grandiflora ( Sars, 1856) View in CoL : Verrill 1879a: 199; 1879b: 32; 1883: 40, Pl. IV Figs. 6, 6 View FIGURE 6 a; 1885: 535; Storm 1896: XXI; Whiteaves 1901: 32; (part) Broch 1912b: 5 –9, Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ; (?part) Molander 1918c: 6 –8, Fig. 1 View FIGURE 1 ;? Kükenthal 1919: 17, 19, 26, 43–44, 672, 681–685, 730, 788, 796, Figs. 17 View FIGURE 17 , 315; (part) Verrill 1922: 18 –19, Fig. 2 View FIGURE 2 , Pl. VI Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 ;? Kükenthal 1924: 14 –16, Figs. 13–14 View FIGURE 13 View FIGURE 14 ; (part) Thomson 1927: 16 –18, Pl. I Fig. 20 View FIGURE 20 , Pl. III Fig. 34 View FIGURE 34 , Pl. IV Figs. 6 View FIGURE 6 , 16 View FIGURE 16 , Pl. V Fig. 28 View FIGURE 28 ; Molander 1929: 35 –37; Thomson 1929: 4; Aurivillius 1931: 10;? Deichmann 1936: 75, 78–79; (part) Stiasny 1937: 20 –23, Figs. F1, F2, Pl. I Figs. 6 View FIGURE 6 , 7 View FIGURE 7 ;? Verseveldt 1940: 37 –47, Figs. 13–15 View FIGURE 13 View FIGURE 14 View FIGURE 15 ; (part) Madsen 1944: 32 –33, Fig. 32 View FIGURE 32 ; Carlgren 1945: 33 – 34, Fig. 8 View FIGURE 8 ; Bayer 1956b: F194, Fig. 140,3; Bayer 1961: 67 –68; Tixier-Durivault & d'Hondt 1974: 1393; Grasshoff 1981: 745, Karte 1, 942; Carpine & Grasshoff 1985: 11 –12; Verrill in Bayer & Cairns 2004: Pl. 64 Fig. 8, 8 View FIGURE 8 b; Watling & Auster 2005: 292; Arantes & de Medeiros 2006: 11 –17, Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 .
? Gymnosarca bathybius Saville Kent, 1870 : 397, Pl. 21 Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 but see Stiasny 1937: 19.
NOT: Anthothela grandiflora ( Sars, 1856) View in CoL : Möbius 1873: 260 [thought to be Eunephthya View in CoL ?] see Madsen 1944
NOT: Anthothela grandiflora ( Sars, 1856) View in CoL : Storm 1879: 144 [found to be Anthelia fallax Broch, 1912a ]
NOT: Anthothela grandiflora ( Sars, 1856) : Grieg 1890: 11 [thought to be Paramuricea sp.]
NOT: Anthothela grandiflora ( Sars, 1856) : Grieg 1894: 3, Pl. I Figs. 1–2 View FIGURE 1 View FIGURE 2 [found to be Lateothela grandiflora n. comb.]
Material examined. Holotype: NHM, UIOslo B1365, Öxford, Finmark, northern Norway, depth 365 m, no date recorded; ZMUC ANT- 000470 , same data as holotype and labelled as a “co-type”.
Other material: NTNU-VM 63139 View Materials , Agdeneståa, Trondheimsfjord, Norway, 63.646°N, 9.752°E, depth 100– 250 m, Torkild Bakken, 21st June 2001 GoogleMaps ; NTNU-VM 63141 View Materials , Rødberg , Trondheimsfjord, Norway, 63.468°N, 9.999°E, depth 200–300 m, Torkild Bakken, 5th December 2006 GoogleMaps ; NTNU-VM 40341 View Materials (part), Dyrviknes 27, Trondheimsfjord, Norway, 63.603°N, 9.757°E, depth 120 m, 18th May 1965 GoogleMaps ; NTNU-VM 67148 View Materials & 67149, Brettingen , Trondheimsfjord, Norway, RV Gunnerus, stn. 2011022, 63.659°N, 9.798°E, depth 200– 100 m, Torkild Bakken, 14th June 2011 GoogleMaps ; NTNU-VM 40338 & 40339, unknown locality, determined by Broch 1912; ZMUB 17759 View Materials (part), Skarnsundet , Trondheimsfjord, Norway, August 1899 ; ZMUB 12187 View Materials , Molde , Julneset, Midfjord, Norway, RV G.O.Sars, stn. 101, depth 275 m ; ZMUB 60328 View Materials , Langenuen , Klinkholmen, Tysnes, Norway, August 1894 ; ZMUB 455 View Materials , Haakonsund , Norway, determined by Danielessen & Koren ; NHM, UIOslo B1366, Selsövik , Norway, depth 182 m; ZMUC-ANT-000470, Brettingsnes , Trondhjemsfjord, Norway, depth 150 m, 21st September 1934 ; ZMUC-ANT-000469, Rødberg , Trondhjemsfjord, Norway, depth 150–300 m, 17th July 1911 ; ZMB 5527 View Materials (part), Rødberg , Trondheimsfjord, Norway, depth 300–350 m, 1913 ; MCZ 51047, Banquereau Bank , off Nova Scotia, Canada, U. S. Fish Commission no. 5705, Gloucester Fisheries Lot 418, 44.217°N, 58.033°W, depth 320 m, 1973 GoogleMaps ; MCZ 51048, unknown locality; MCZ 50734, Browns Bank , east coast of Canada, schooner Chester B. Lawrence, 42.517°N, 64.333°W, depth 300 f (feet or fathoms), Capt. Wm. H. Greenleaf GoogleMaps ; MOVI 20919 View Materials , east coast of Rio Grande do Sul, Brazil, 34.324°S, 51.572°W, depth 822 m, 5th March 2002 GoogleMaps ; unregistered specimen, NEREIDA 0 610, zone NAFO, tow DR72, 46.0239°N, 46.686°W, depth 710 m, collected by Tina Molodtsova thanks to Mar Sacau and Javier Murillo Perez (IEO, Vigo, Spain) and sampling program NEREIDA, 28th June 2010 GoogleMaps .
Description:
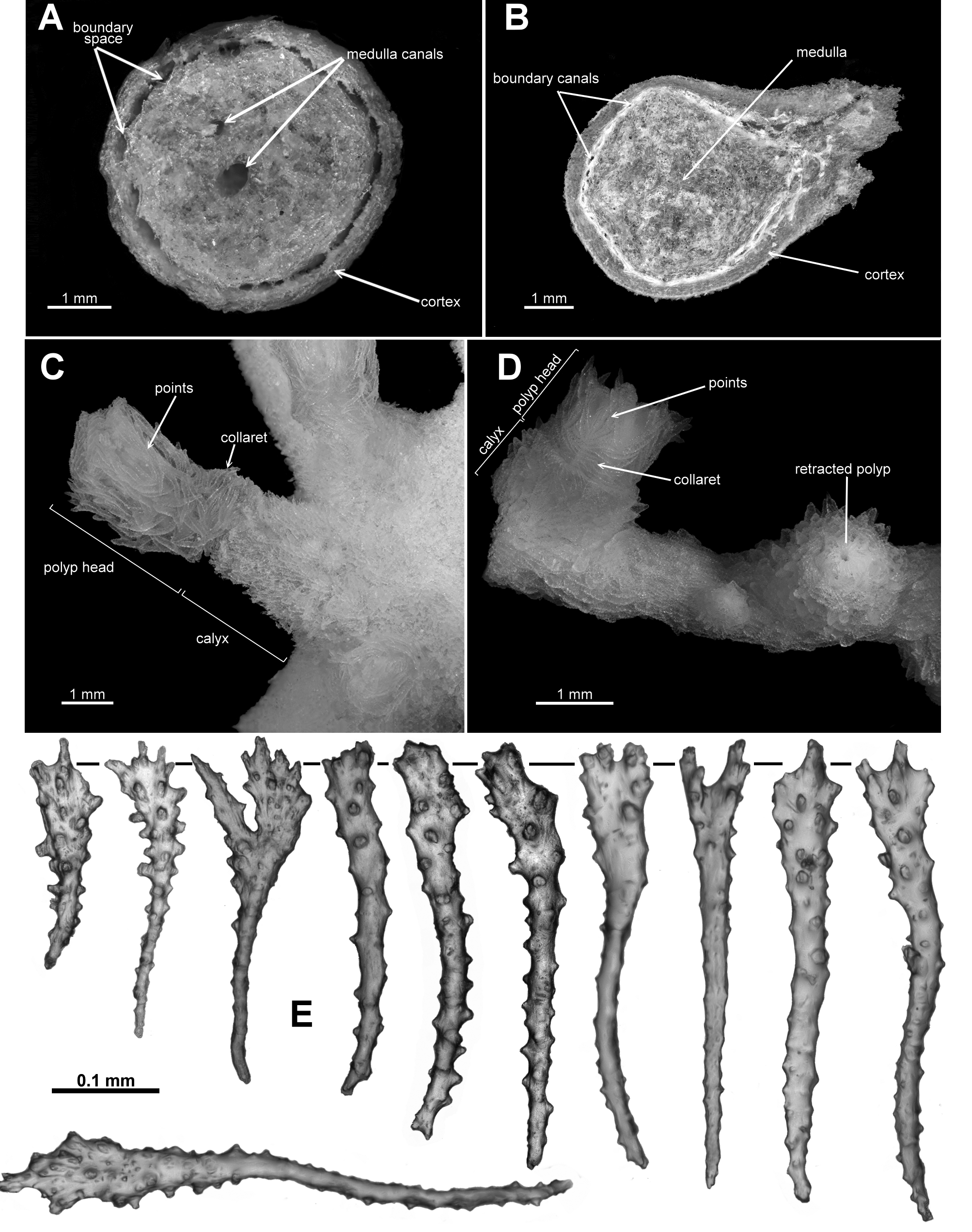
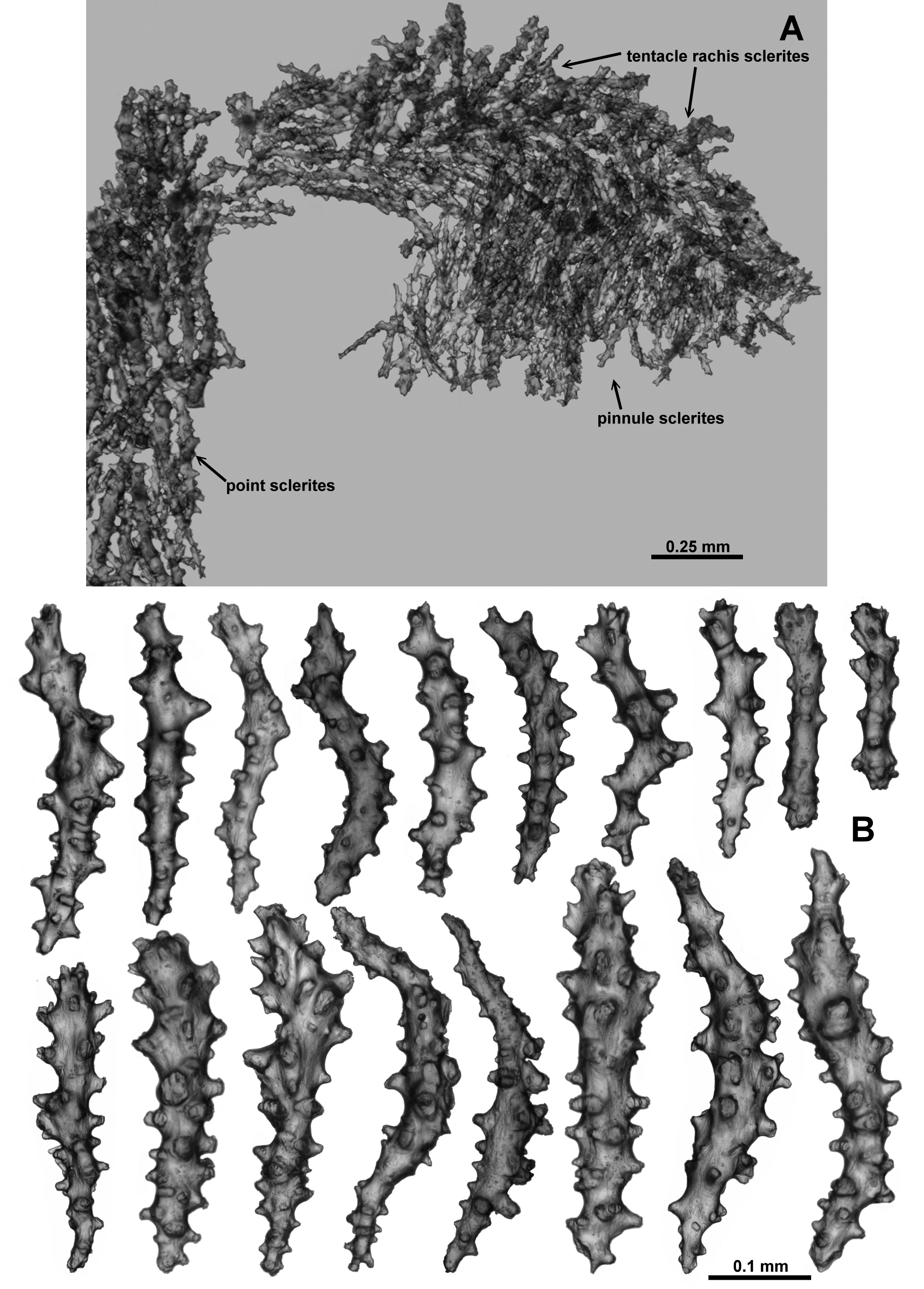
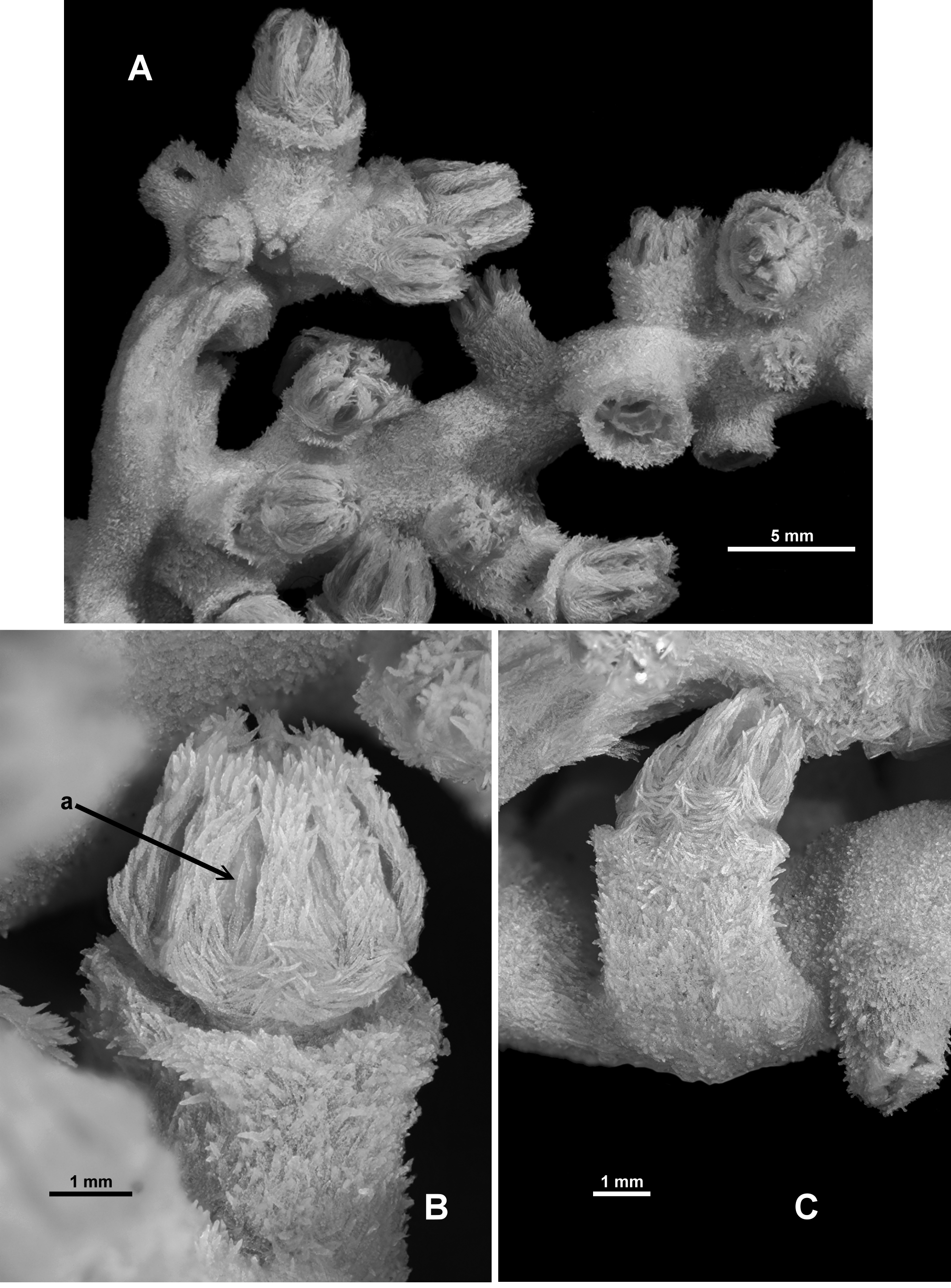
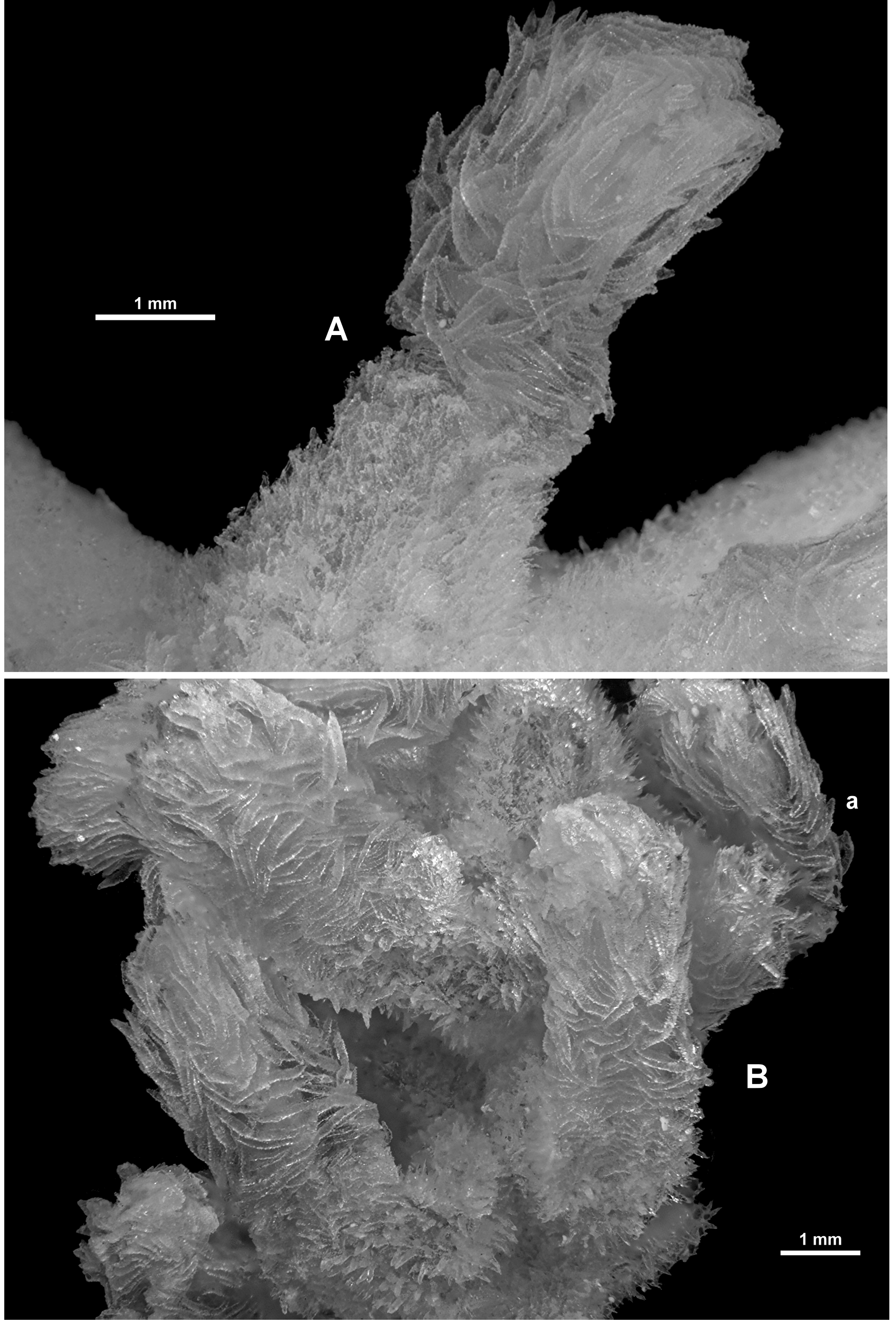
Colony form: The holotype is made up of many fragments of a tangled colony which once was described as “die Grösse eines Menschenkopfes” (translated as “the size of a human head”) ( Broch 1912b) ( Fig. 4 View FIGURE 4 A). There is no central trunk or evidence of main branches and there is no consistent arrangement of branching. In Sars’ original description, he made it clear (“D'ailleurs il n'y a aucune différence entre les tiges et les branches: elles ont la même apparence, la même forme, la même grosseur“) that there is no difference between stems or branches: they have the same appearance, same form and same size. Many of the fragments are small pieces of narrow branches which are often twisted and tangled so it is impossible to reconstruct the colony into the original shape. There are also multiple anastomoses and bifurcations throughout the colony fragments ( Fig. 4 View FIGURE 4 B). Examples of membranous portions of the colony are evident including the holdfast which is encrusting a piece of coral rubble. Several branches emanate from the membranous parts of the colony. Most branches are of a relatively uniform diameter (approximately 2.2–3.4 mm), although there are some outside this range ( 1.3–4.2 mm), and they are basically circular in cross-section, although calyces and bifurcation points tend to cause some distortion.
Calyces occur with no apparent order along and all around the branches throughout the colony. The greatest distance between calyces is approximately 12 mm although they are more commonly closer together. Tight bunches of calyces occur on the branch tips where there is very little or no space between them ( Fig. 5 View FIGURE 5 A). Isolated calyces are also evident on the membranous parts of the colony.
The colony is in good condition, albeit in many fragments, with many polyps still attached and, in general, the cortex is complete.
Colour: In the original description no mention was made regarding the live colour, although Sars did comment that the medulla is a darker colour than the cortex. The holotype is now light brown to cream in alcohol. Other specimens recently collected are creamy pink ( Fig. 13 View FIGURE 13 B, C).
Polyps and calyces: Calyces are tall, flat-topped, robust cylinders which protrude basically at right angles from the branches ( Fig. 5 View FIGURE 5 B). They are usually between 2.2–3.2 mm high although there are some larger ones up to 4.5 mm. Polyps may extend 1.4–2.6 mm above the lip of the calyx and are approximately 2–3mm wide. Calyces and polyps are usually taller than they are wide. Sars considered the relatively large size of the calyces and polyp as a distinguishing factor, mentioning that the height of the “cellules polypifères” is generally twice the diameter of the branches. The calyces do not have obvious longitudinal ridges although there can be slight bulges where the base of the tentacles meet the calyx lip. Most of the polyps are partly retracted such that the base of the polyp head rests on the lip of the calyx and the polyp neck is not visible ( Fig. 5 View FIGURE 5 B) but there are some examples where polyps are fully retracted into their calyx ( Fig. 5 View FIGURE 5 Aa) despite Sars stating that he did not observe any in this state. Occasionally fully exsert polyps occur, with the polyp neck visible—a polyp and calyx combined then being up to 6.5 mm high. All polyp heads are well protected by crowded sclerites arranged as points and an indistinct collaret, and the visible polyp necks are also covered in sclerites. The tentacles fold into the polyp mouth so the polyp heads are rounded mounds with eight distinct furrows ( Fig. 5 View FIGURE 5 B). There are approximately 12 pinnules along each side of the tentacles with a fan of pinnules around the tip.
Medulla and Cortex: The central medulla is made up of tightly packed, longitudinally and obliquely placed sclerites, surrounded by a thin cortex which is separated from the medulla by a ring of longitudinal boundary canals ( Fig. 5 View FIGURE 5 C). The canals run parallel and close together throughout the colony and remain identifiable as individual canals. They do not extensively anastomose or form a boundary space as such but do provide a clear separation of the medulla from the cortex. In the thickest branches, presumably older and from closer to the base of the colony, there may be some patches where the density of medulla sclerites lessens. These patches appear as indistinct canals in the central medulla ( Fig. 5 View FIGURE 5 D) and are easily deformed or obscured by sclerites during the making of a crosssection. In thinner branches, presumably younger and farther from the base of the colony, the ill-defined canals in the centre of the medulla are only occasionally visible in cross-section.
For polyps along the branches, body cavities truncate abruptly forming a flat base where they abut the medulla, while polyps which are clumped at the branch tips have a body cavity which extends somewhat deeper within the branch, eventually finishing at the start of the medulla proper.
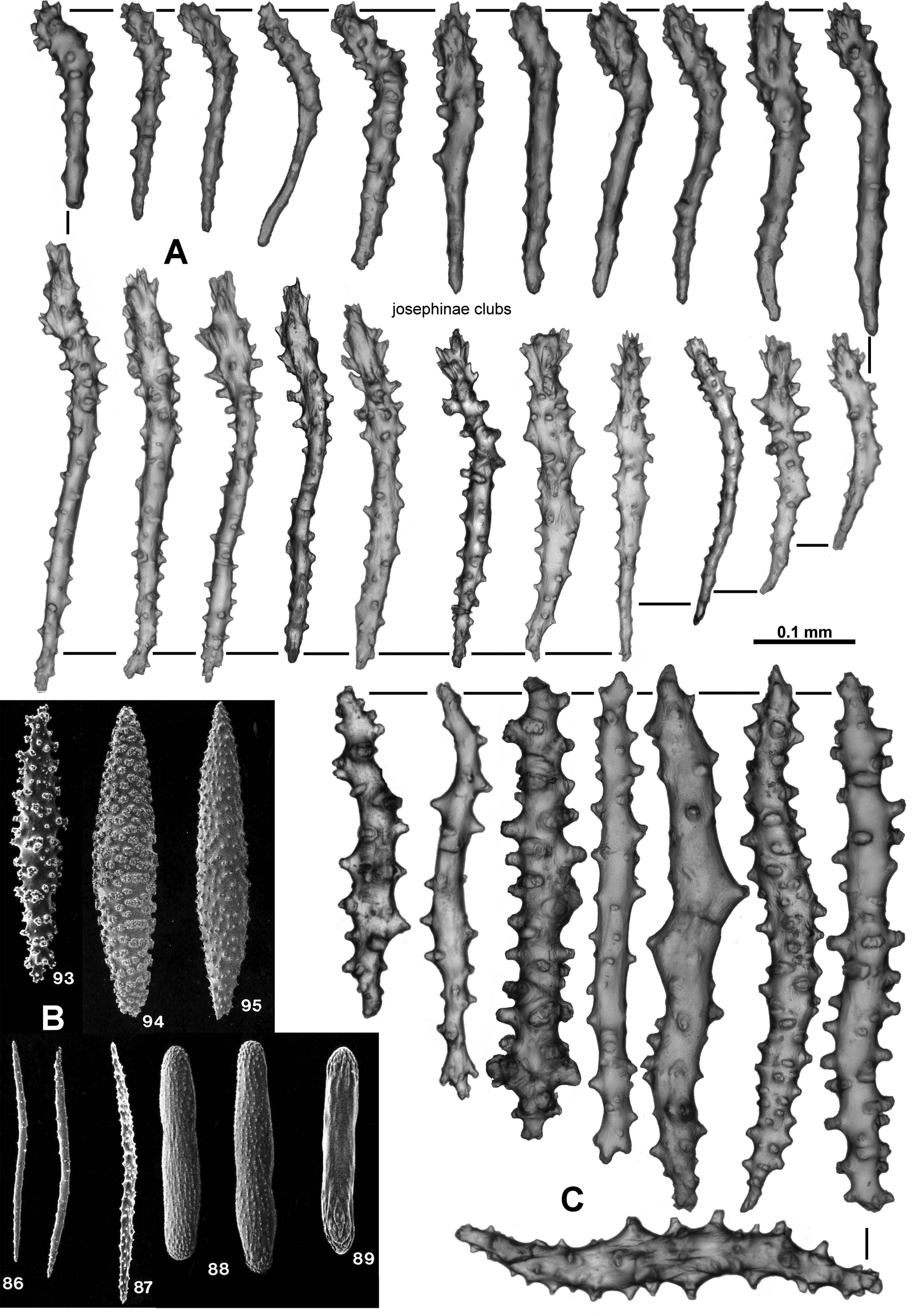
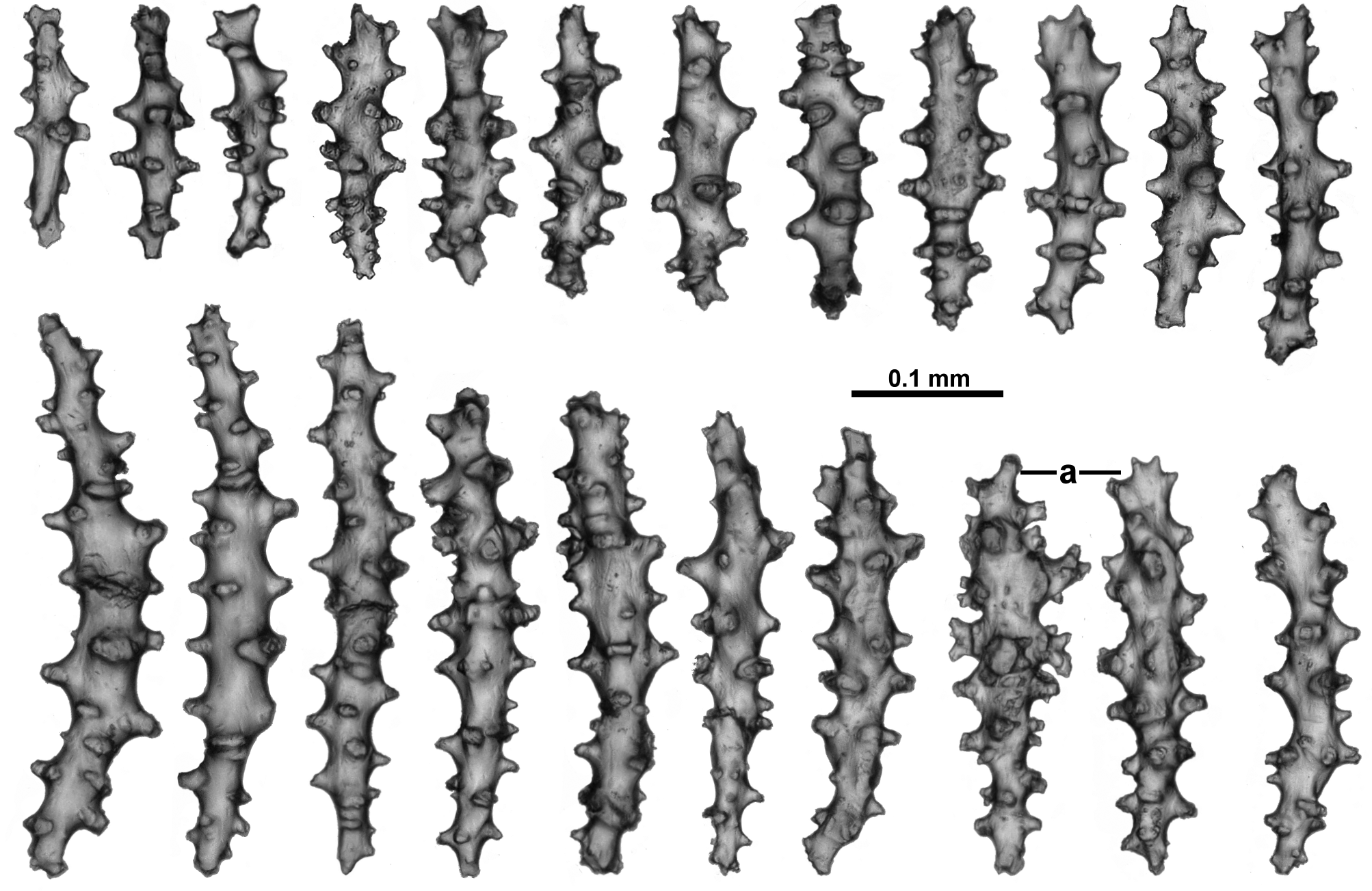
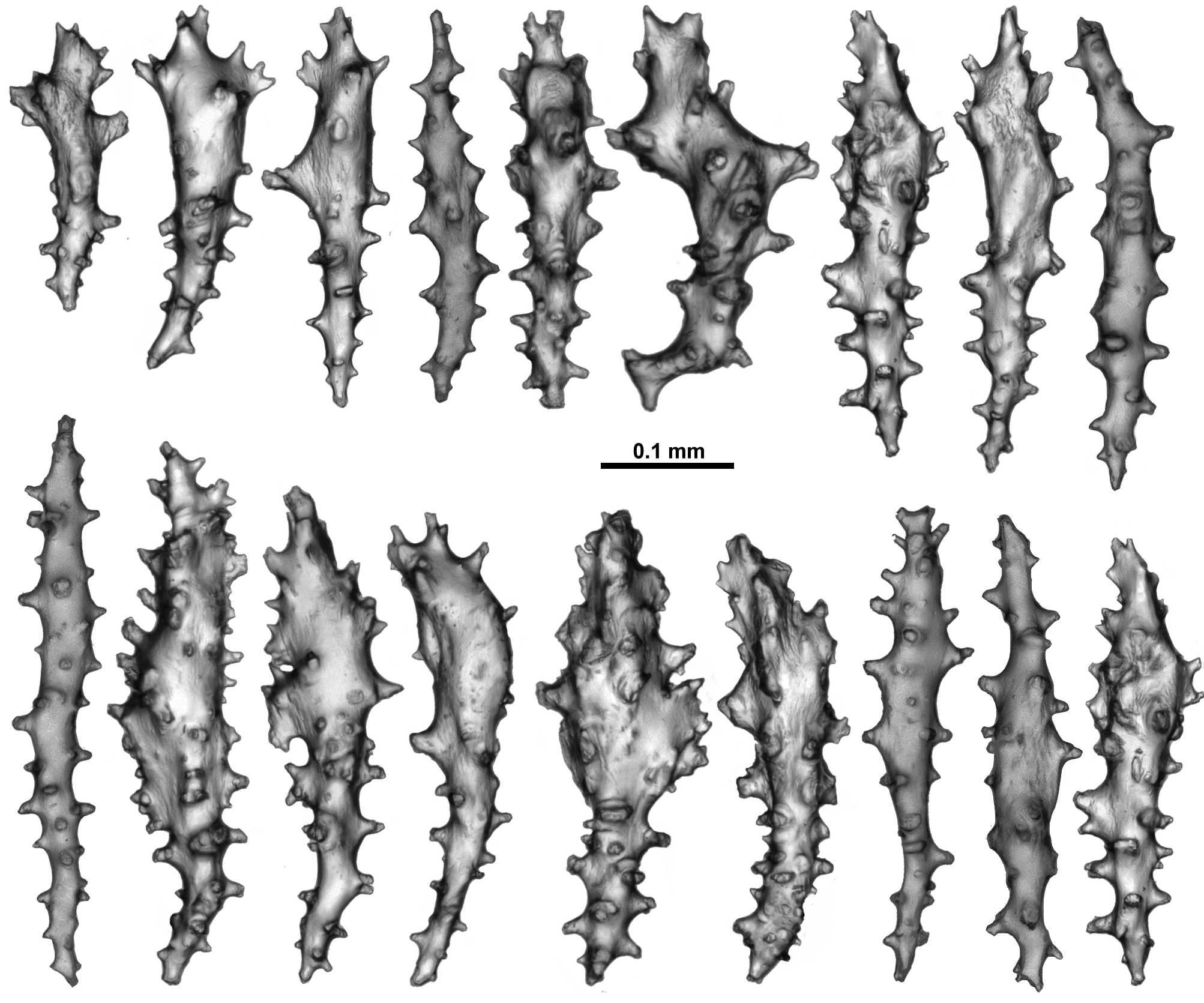
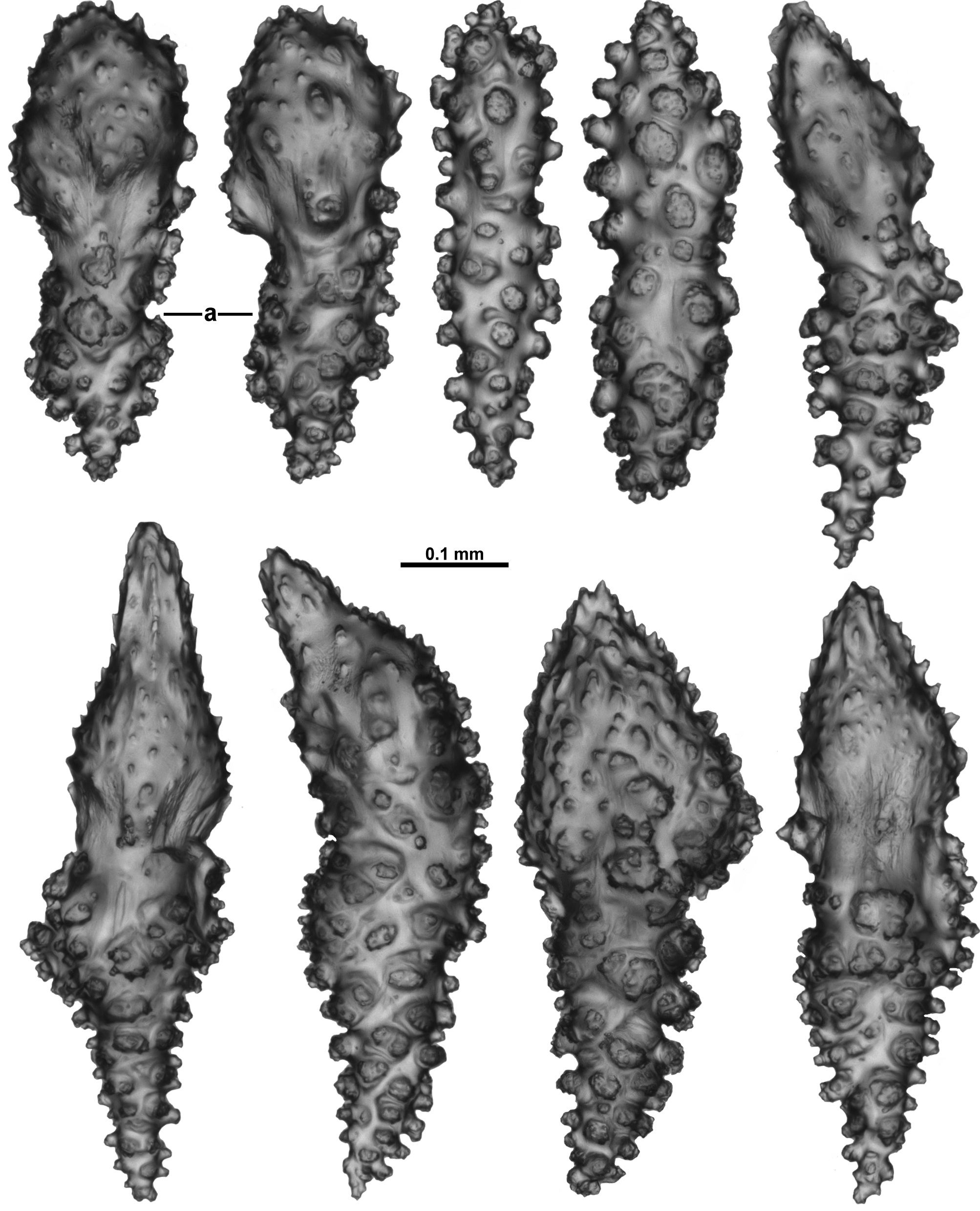
Sclerites: Polyps, calyces and cortex are all covered in crowded sclerites. On the polyp head transversely arranged sclerites form an indistinct collaret approximately 8 sclerites deep, while above they are arranged en chevron grading to longitudinal in the points ( Fig. 5 View FIGURE 5 B). These sclerites are mostly straight or slightly curved sticks and spindles with simple tubercles ( Fig. 6 View FIGURE 6 ). Sizes can range from 0.4–1.3 mm but most are between 0.45–0.85 mm. Some sclerites have the distal tip with mildly more developed tubercles or spines ( Fig. 6 View FIGURE 6 a). Very occasionally, sclerites can have complex warts. Below the collaret, similar sclerites are arranged obliquely on the polyp neck, sparser than on the polyp head, presumably to allow the polyp to invaginate at the neck area.
Along the aboral side of the tentacle, sclerites are arranged longitudinally ( Fig. 7 View FIGURE 7 A) and are all mostly similar to the sclerites from the points although shorter. They are straight or slightly curved tuberculate sticks and spindles with complex warts occurring occasionally and processes slightly more developed on one end ( Fig. 7 View FIGURE 7 B). Sclerites grade in size from longest ( 0.5 mm) at the proximal end of the tentacle to shortest ( 0.25 mm) at the distal end.
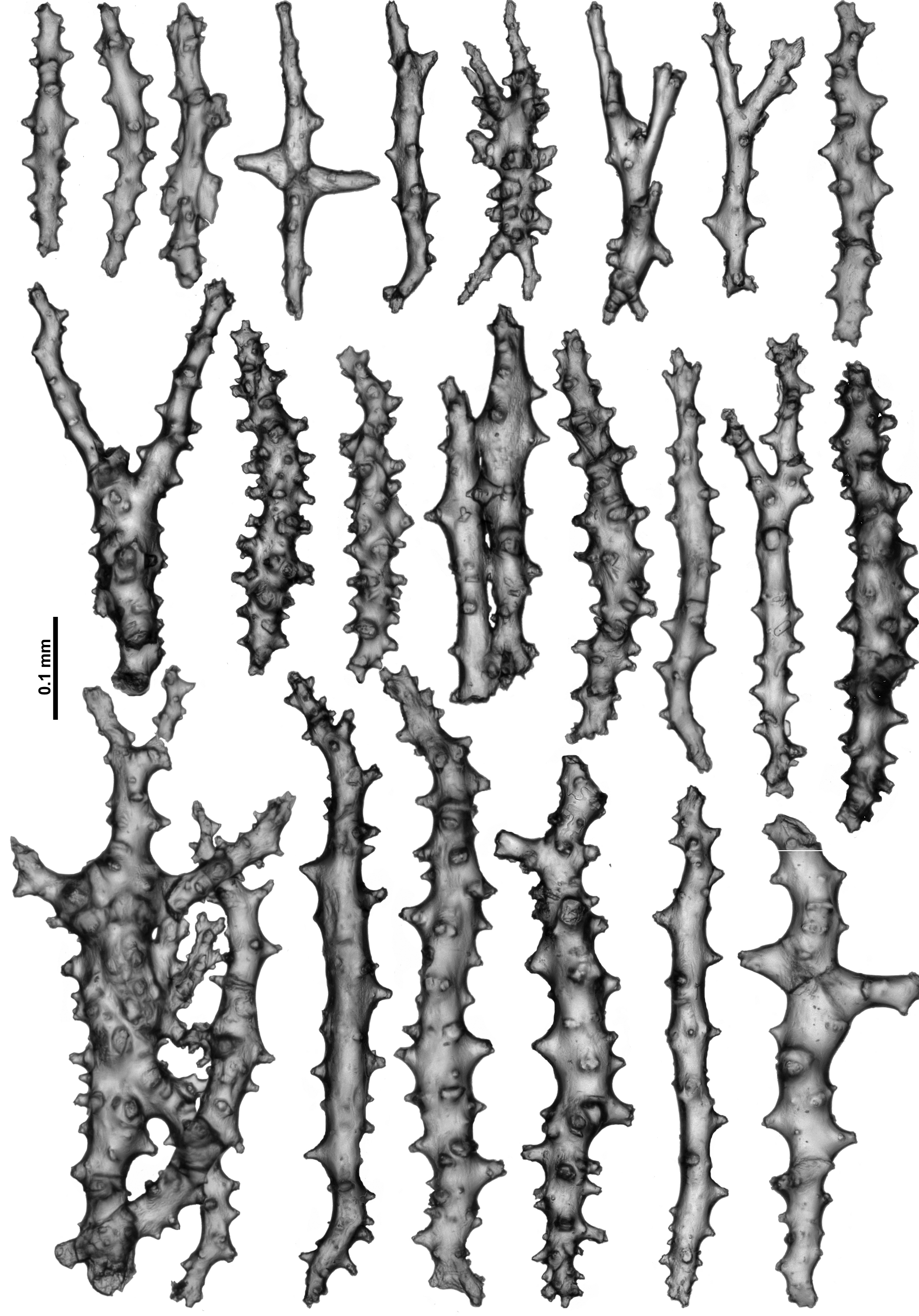
Long, thin, spatulate clubs extend longitudinally most of the way down the pinnules ( Fig. 8 View FIGURE 8 A). These sclerites are crowded, with the spatulate tip toward the distal end of the pinnules, and are easily broken during dissection. The spatulate clubs have long, narrow handles and flattened, occasionally forked, tips and are usually straight but can be curved or with bent tips ( Fig. 8 View FIGURE 8 Ba). There are also short rods with sparse tubercles, narrow sticks and spindles without a flattened tip and very small spindles in the pinnules ( Fig. 8 View FIGURE 8 Bb). The size of the spatulate clubs ranges from 0.34–0.5 mm while the smaller rods and spindles are approximately 0.08–0.21 mm long.
Small rods ( 0.08–0.24 mm long) with cone-like prominences and sparse warts occur in the pharynx ( Fig. 9 View FIGURE 9 A). These sclerites are arranged in ill-defined lines in the pharynx corresponding to the mesenterial insertions and are not crowded ( Fig. 9 View FIGURE 9 B).
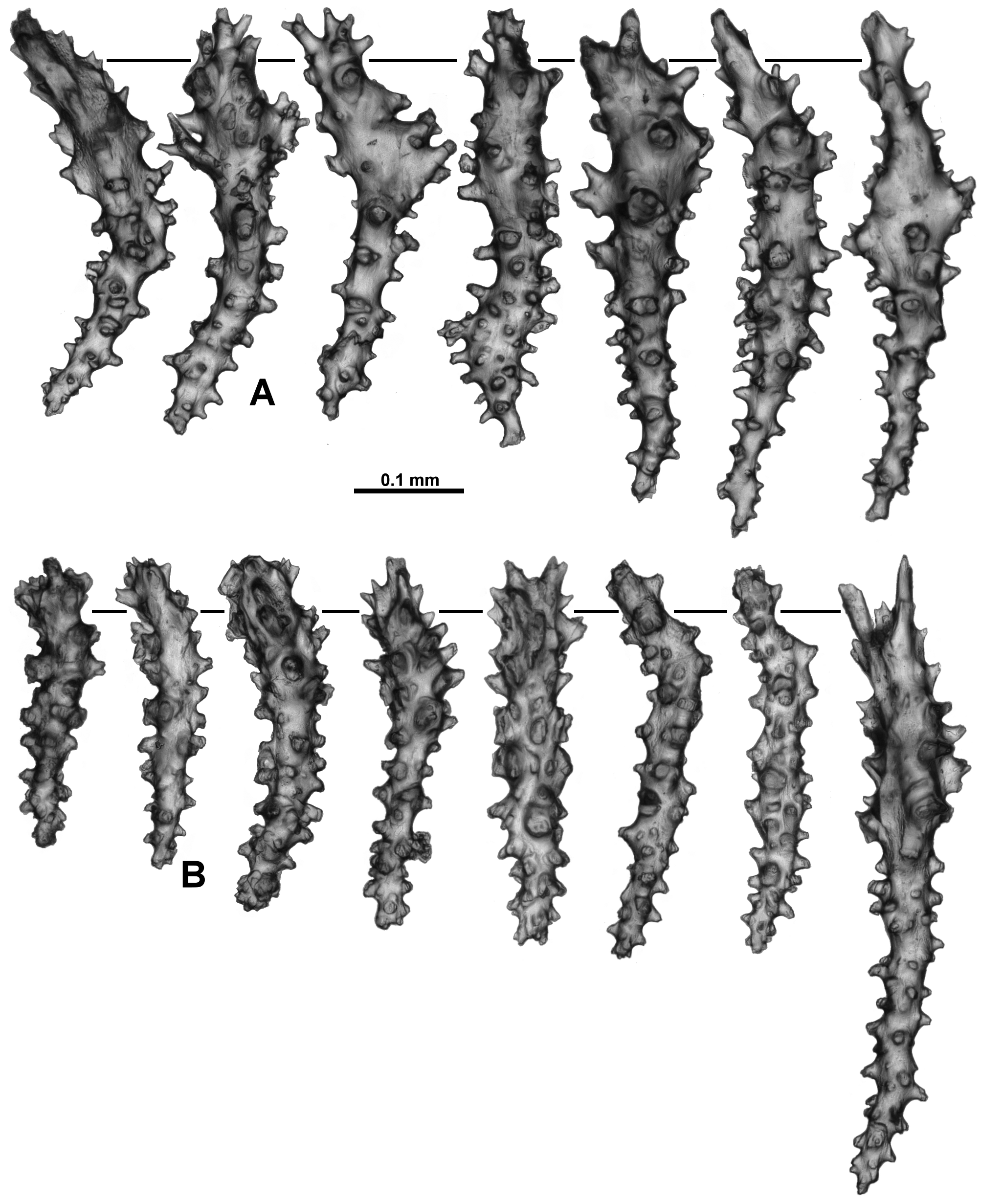
The sclerites of the calyx are arranged in a dense layer, longitudinally to obliquely in the wall. They are short, straight or slightly curved sticks and spindles mostly with simple tubercles ( Fig. 10 View FIGURE 10 ). Occasionally more complex warts occur and some sclerites have a clavate tip with slightly flared or foliose spines ( Fig. 10 View FIGURE 10 a) but they do not form true thorn clubs. The sclerites have a small size range, only varying from approximately 0.23–0.45 mm long.
The sclerites from the cortex are similar to those in the calyces—small, straight, tuberculate sticks and spindles of a fairly uniform shape and size ( Fig. 11 View FIGURE 11 ). Most of the sclerites are between 0.16 and 0.38 mm in length but slightly longer sclerites also occur. Occasionally, sclerites with more complex warts occur but more commonly the tubercles are simple and relatively sparse. Some sclerites can have one marginally more complex tip making them slightly clavate ( Fig. 11 View FIGURE 11 a).
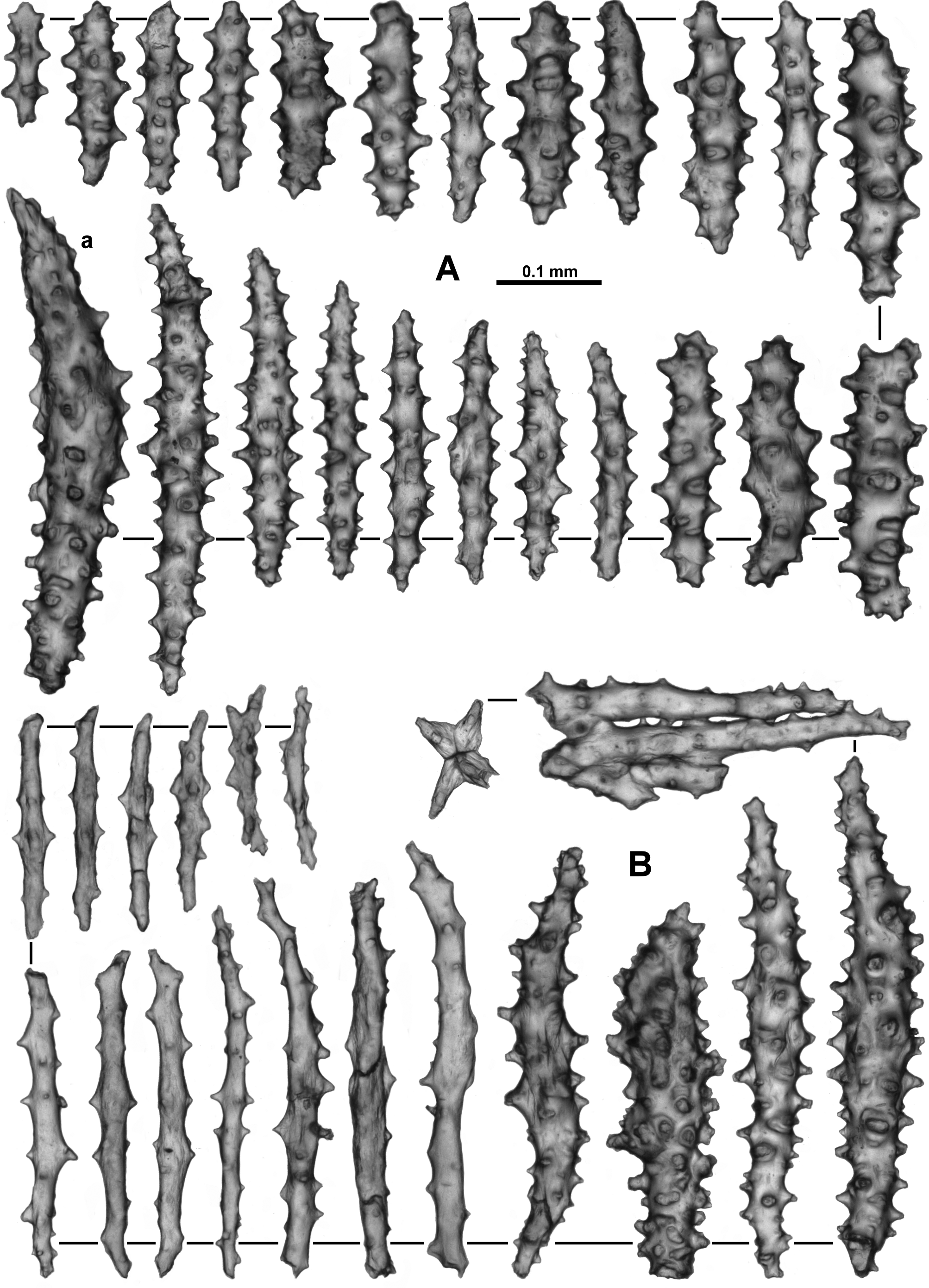
The medulla is composed of tightly packed sclerites, mainly sticks and spindles with simple to complex tubercles ( Fig. 12 View FIGURE 12 ). There are examples of fusion, branching and forking among the sclerites resulting in quite complex forms. The length of the sclerites can vary considerably ( 0.2–0.9 mm) and the longest are probably underrepresented in the figure as it is difficult to sample them without breakage.
All sclerites are transparent and colourless under transmitted light.
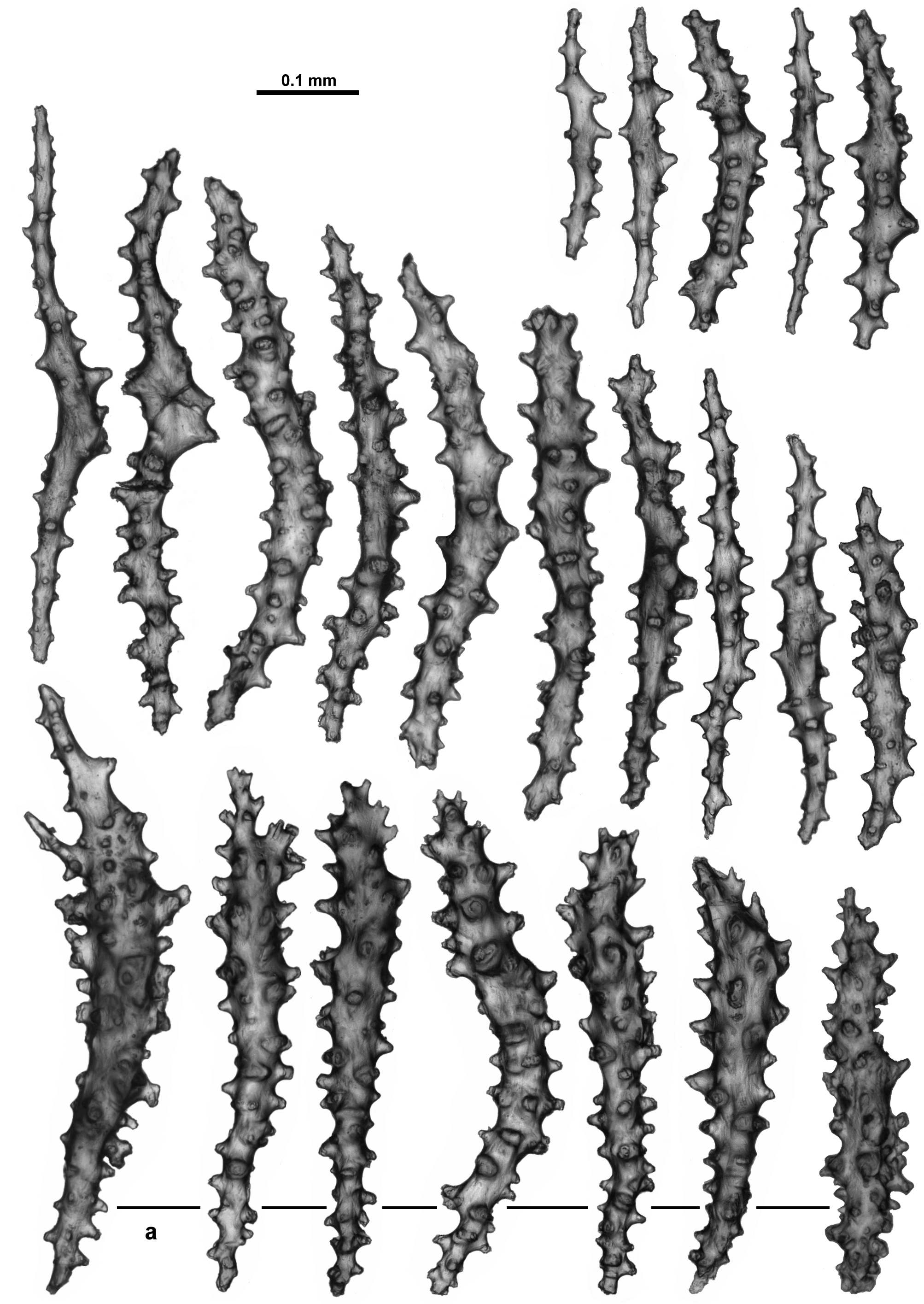
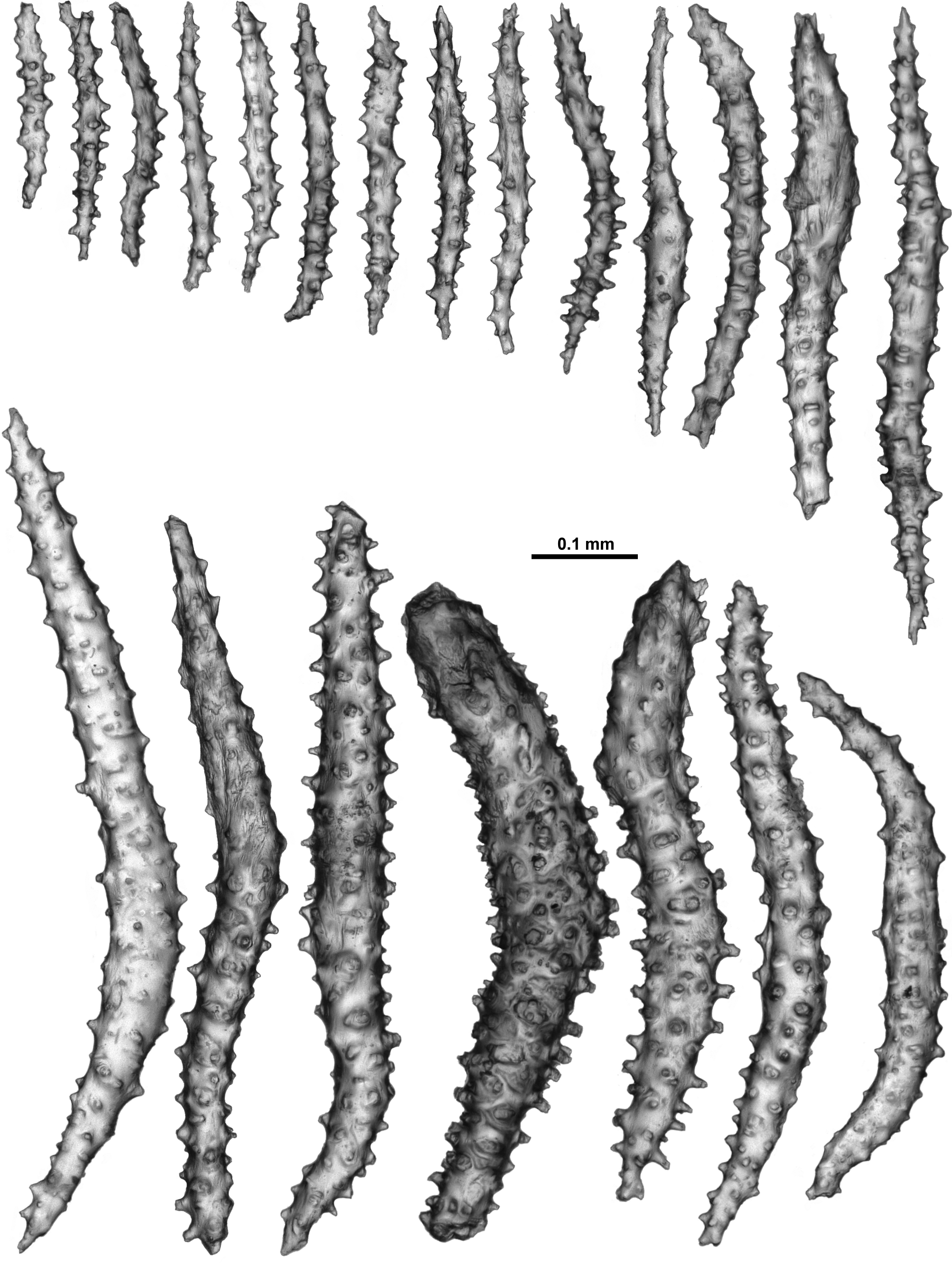
Variability: Variability of sclerite development within this species appears to be quite substantial. The holotype is from the northern tip of Norway, at the extreme northern point of the distribution of the specimens examined, and it is a specimen with a very consistent form of sclerites (sticks and spindles with simple tubercles) from the aboral side of the tentacles, points, collaret, calyx and the cortex (unfortunately no specimens which are geographically close to the holotype were available). Other specimens have considerable variation in the complexity and shape of the sclerites. One specimen, NTNU-VM 67149, from Trondheimsfjord, Norway, has sclerites from the calyx which are more often club-shaped with very complex warts and spines in addition to the sticks and spindles with simple tubercles like those in the holotype ( Fig. 13 View FIGURE 13 A). Such complex sclerites in the calyx are common in other specimens investigated, particularly in specimens from the Trondheimsfjord, yet the colony, polyp form and the other sclerites are similar to those in the holotype. Photographs of two specimens from Trondheimsfjord taken soon after collection (NTNU-VM 67149 and NTNU-VM 67148) demonstrate the live colour to be creamy pink ( Fig. 13 View FIGURE 13 B, C). In addition, there are many specimens with more complex and larger sclerites from the tentacles, points and surface than those observed in the holotype. In particular, despite much similarity with the holotype in other areas, the sclerites from the tentacles of ZMUC-ANT-000470 and NTNU-VM 40338 have more developed processes on the distal tips ( Fig. 14 View FIGURE 14 A, B). At this stage, it is assumed they are more complex versions of those in the tentacle rachis of the holotype, although further collections may allow a definitive delimitation within these degrees of complexity.
Similarly, the colony surface of sample NTNU-VM 63139, along with small sticks and spindles like those of the holotype, has numerous short, straight, club-shaped forms with smooth areas as well as more developed spines and warts ( Fig. 15 View FIGURE 15 ). These surface sclerites were recorded in lesser amounts in other specimens as well, but not the holotype.
Distribution: Confirmed records are from the north eastern Atlantic Ocean in deep coastal waters and fjords of Norway and Iceland; north western Atlantic Ocean, in deep waters off the coast of Canada and USA; the Gulf of Biscay, off the west coast of France; and the south western Atlantic Ocean off the coast of southern Brazil.
Depth: Confirmed specimens 100–960 metres; most commonly between 100–500 metres.
Remarks: There has been much confusion over many years between A. grandiflora and material that has herein been assigned to the new genus Lateothela . In every collection examined which contained specimens of A. grandiflora , specimens of Lateothela n. gen. were also found, almost always erroneously determined as A. grandiflora . In Broch’s (1912b) extensive re-description of A. grandiflora it now seems clear that he included more than one specimen in his description, and at least one of these was likely to have been a specimen of Lateothela grandiflora n. comb. The figure of the small, warty rodlets from the calyces and cortex ( Broch 1912b Fig. 2a View FIGURE 2 , b), which are common in L. grandiflora n. comb. but do not occur in any significant number in A. grandiflora , perpetuated the confusion between the two genera. One of the chief morphological differences between these two species is the preponderance of these rodlets in the calyces and cortex of L. grandiflora n. comb. (mixed with tuberculate sticks and spindles) compared with their rarity in A. grandiflora . Other differences between the two species are colony form and differences in the pinnule and tentacle sclerites.
Much of the subsequent literature perpetuated Broch’s (1912b) incorrect description, thus some later determinations and descriptions of specimens are clearly incorrect or cannot be confirmed ( Verrill 1922; Stiasny 1937; Verseveldt 1940; Madsen 1944) and many specimens remain incorrectly identified. For example, Verrill (1922) when describing A. grandiflora stated the figures are “from the type described in 1869”, but Plate VI Fig. 1 View FIGURE 1 in particular, and his description of the appearance of the calyces, appears to depict L. grandiflora n. comb. with the small warty rodlets in the calyx and surface. However, the colony depicted in Verrill’s Text Figure 2 View FIGURE 2 is more like the holotype of A. grandiflora and not of a colony of L. grandiflora n. comb. There is no way to confirm exactly which specimen (or specimens?) Verrill figured in this paper, however it is possible it may not have been the holotype of A. grandiflora , or perhaps he used more than one specimen to assemble the description. Additionally, this current research has confirmed that of the specimens listed as A. grandiflora in Madsen (1944) three are valid, but one specimen (‘Thor’ Stat 168) is an example of L. grandiflora n. comb. —another instance of the confusion of the two species.
The variation observed amongst specimens of A. grandiflora shows the holotype to be a specimen with minimal complexity in the warts and tubercles of the sclerites and in the shape of the sclerites themselves. Nevertheless, the tangled colony form, long thin spatulate clubs in the pinnules and generally simple tuberculate sticks and spindles with a tendency to be clavate seem to be consistent across the specimens examined. At this stage there are not enough consistent differences to reliably delimit other species within the sample set, however, considering the large geographic range which is currently recorded for this species it would not be surprising if future studies can confidently define other Anthothela species within this group.
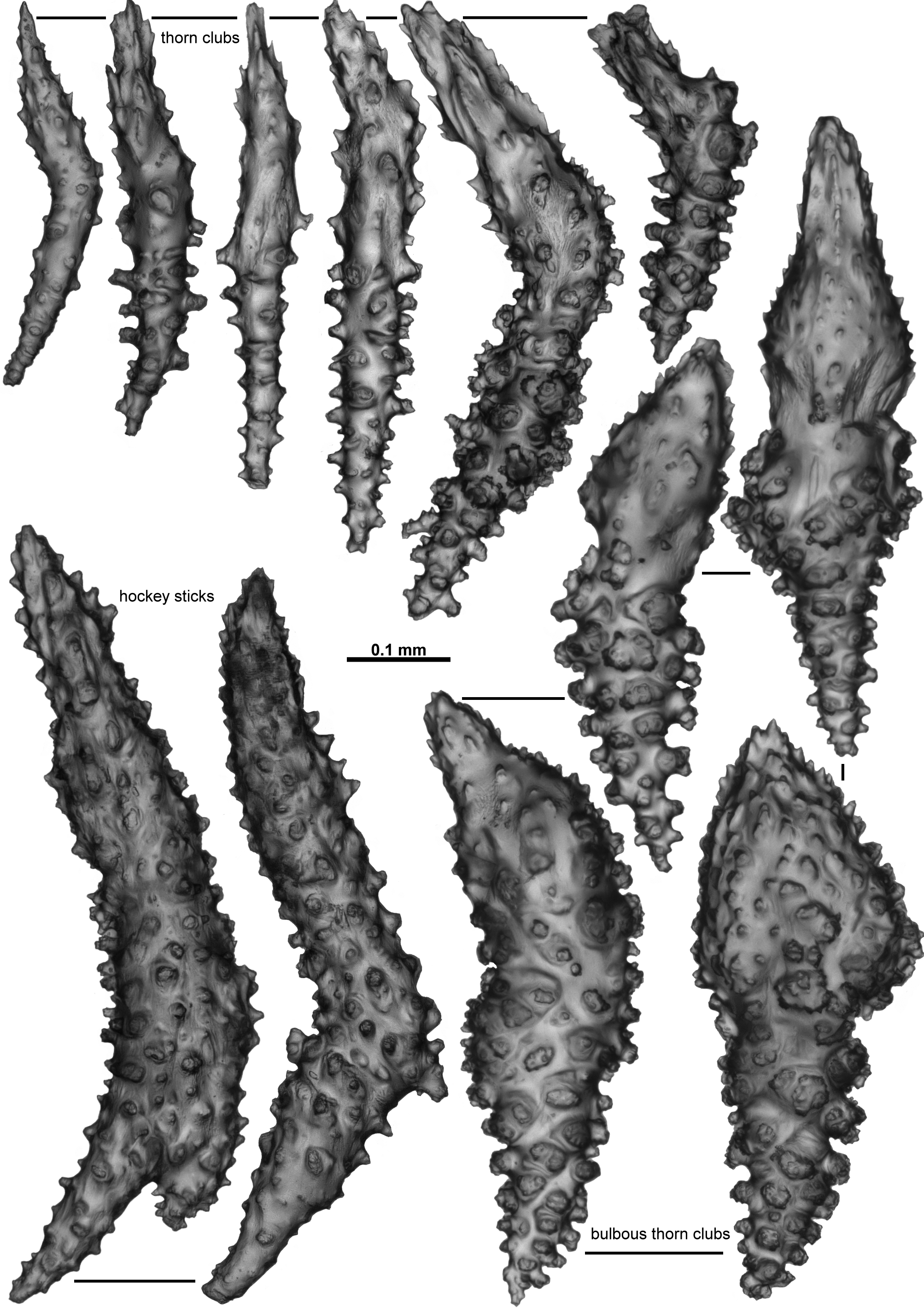
A. grandiflora as detailed here appears to be reasonably wide-spread in the deep waters of the Atlantic Ocean. Anthothela tropicalis and Anthothela quattriniae n. sp. also occur in the Atlantic, although A. quattriniae n. sp. is currently only recorded from the Gulf of Mexico. Both of these species have large thorn clubs, which are thorny in A. tropicalis ( Fig. 38 View FIGURE 38 ) and bulbous in A. quattriniae n. sp. ( Fig. 63 View FIGURE 63 ), and both have colonies with very prickly surfaces with the thorn clubs projecting outwards. A. grandiflora lacks true thorn clubs and has a relatively smooth surface. Anthothela pacifica has currently only been recorded from the northern Pacific Ocean, and has small, straight sticks, spindles and clubs in the calyces and cortex which are smaller than those recorded in A. grandiflora . Specimens of another species herein assigned to Anthothela , A. vickersi n. comb., carry the same haplotype as specimens of A. grandiflora using two mitochondrial gene regions ( mtMutS and igr1– cox1). However, the morphological and geographical differences between these populations were deemed enough to maintain separation into two species. Chiefly, the sclerites in a calyx of A. vickersi n. comb. are large, bent thorn clubs which project out from the calyx wall ( Figs. 21 View FIGURE 21 ; 27), while the smaller sclerites in a calyx of A. grandiflora , although at times complex, do not consistently have spear tips which project out of the colony. Additionally A. vickersi n. comb. has short, relatively broad, tuberculate rods that are common in the cortex ( Fig. 31 View FIGURE 31 ), and long, narrow sticks and spindles in the tentacle rachis and pinnules ( Fig. 25 View FIGURE 25 ), both of which are not common in A. grandiflora ; the branches of the colonies of A. vickersi n. comb. are not as narrow or flexible as A. grandiflora ; and A. vickersi n. comb. has been recorded from southern Australia and New Zealand while A. grandiflora is only known from the Atlantic Ocean.
Anthothela aldersladei n. sp. from Western Australia differs from A. grandiflora by having short, bent, spiky thorn clubs in the calyces and cortex, giving the colony a very spiky appearance, and has very large sclerites (relative to the polyp head) in the points ( Figs. 43 View FIGURE 43 ; 46).
| NHM |
University of Nottingham |
| ZMUC |
Zoological Museum, University of Copenhagen |
| NTNU-VM |
Norwegian University of Science and Technology - University Museum |
| ZMUB |
Museum of Zoology at the University of Bergen, Vertebrate collections |
| ZMB |
Museum f�r Naturkunde Berlin (Zoological Collections) |
| MCZ |
Museum of Comparative Zoology |
| MOVI |
Museu Oceanografico do Vale do Itajai |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Octocorallia |
|
Order |
|
|
SubOrder |
Scleraxonia |
|
Family |
|
|
Genus |
Anthothela grandiflora ( Sars, 1856 )
| Moore, Kirrily M., Alderslade, Philip & Miller, Karen J. 2017 |
Anthothela insignis
| Verrill 1879: 15 |
Anthothela grandiflora ( Sars, 1856 )
| Medeiros 2006: 11 |
| Watling 2005: 292 |
| Carpine 1985: 11 |
| Grasshoff 1981: 745 |
| Tixier-Durivault 1974: 1393 |
| Bayer 1961: 67 |
| Carlgren 1945: 33 |
| Madsen 1944: 32 |
| Verseveldt 1940: 37 |
| Stiasny 1937: 20 |
| Deichmann 1936: 75 |
| Aurivillius 1931: 10 |
| Molander 1929: 35 |
| Thomson 1929: 4 |
| Thomson 1927: 16 |
| Kukenthal 1924: 14 |
| Verrill 1922: 18 |
| Kukenthal 1919: 17 |
| Molander 1918: 6 |
| Broch 1912: 5 |
| Whiteaves 1901: 32 |
| Verrill 1879: 199 |
Gymnosarca bathybius Saville Kent , 1870
| Stiasny 1937: 19 |
| Saville 1870: 397 |
Briareum grandiflorum
| Sars 1856: 63 |