Ameerega altamazonica, Twomey, Evan & Brown, Jason L., 2008
|
publication ID |
https://doi.org/ 10.5281/zenodo.181829 |
|
DOI |
https://doi.org/10.5281/zenodo.6493693 |
|
persistent identifier |
https://treatment.plazi.org/id/2A35E164-FFE7-6652-FF6C-7A9A24A5F9A6 |
|
treatment provided by |
Plazi |
|
scientific name |
Ameerega altamazonica |
| status |
sp. nov. |
Ameerega altamazonica View in CoL sp. nov.
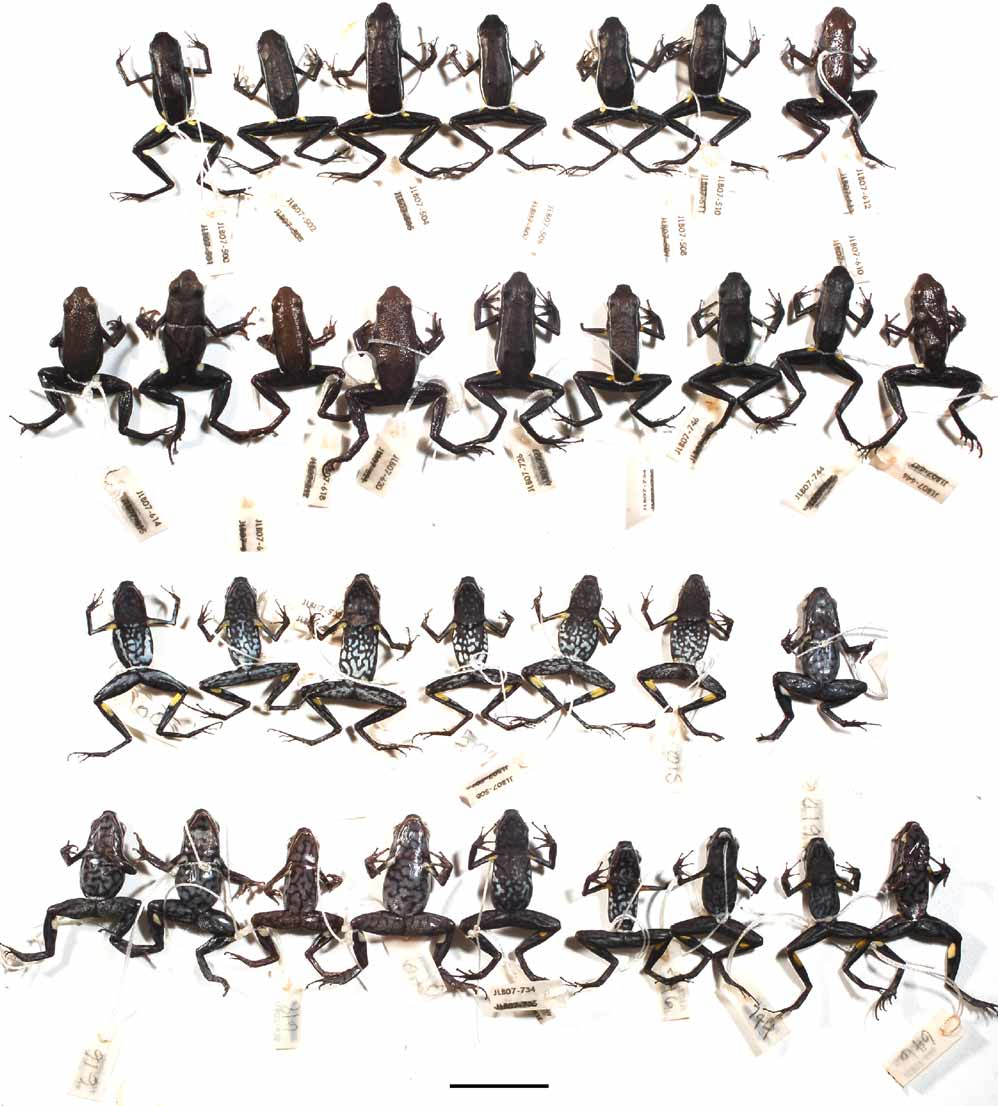
Figures 1 View FIGURE 1 , 2 View FIGURE 2 , 4 View FIGURE 4
Phyllobates pictus View in CoL (non Bibron in Tschudi): Silverstone 1976 p. 40–41, pattern 4 (partim). Dendrobates pictus View in CoL (non Bibron in Tschudi): Myers, Daly, and Malkin 1978 p. 332 (by implication). Epipedobates hahneli View in CoL (non Boulenger): Lötters et al. 1997 p. 33–34, sketch 2 (partim); Schulte 1999: p. 227–237
(partim).
Ameerega hahneli View in CoL (non Boulenger): Grant et al. 2006 (by implication); Lötters et al. 2007: p. 336–342 (partim).
Holotype. MUSM 26937, an adult male that was observed calling, collected by J. Brown, K. Fieselman, and E. Twomey in Departamento San Martín, Perú, 3.5 km N of Tarapoto, Río Shilcayo drainage, 401 m elevation, 6° 27' 44” S, 76° 21' 6” W, 9 June 2007.
Paratopotypes. MUSM 26936, 26938, 26939 collected same date and location as holotype.
Paratypes. All from Peru. San Martín: 20 km SW of Tocache, upper Río Tocache drainage, 865 m elevation, 8°18’32” S, 76°40’33” W (MUSM 24939–24944), collected 11–13 July 2006 by M. Pepper, E. Twomey, and W. Olthof); 7 km NW of Saposoa, Río Shima drainage, 408 m elevation, 6°53’47” S, 76°49’41” W (MUSM 26974), collected 24 June 2007 by J. Brown and E. Twomey); Chazuta, Río Tunumtunumba drainage, 244 m elevation, 6°33’36” S, 76°7’19” W (MUSM 26934, 26935), collected 11–12 June 2007 by E. Twomey); 7 km SW of Huicungo, Río Huayabamba drainage, 352 m elevation, 7°22’3” S, 76°48’48” W (MUSM 26977), collected 25 June 2007 by J. Brown and E. Twomey); 6.5 km N Campanilla, 313 m elevation, 7°25’39” S, 76°39’53” W (MUSM 26981, 26982), collected 25 June 2007 by J. Brown and E. Twomey). Loreto: Pampa Hermosa, west bank of Río Ucayali, 153 m elevation, 7°12’27” S, 75°19’25” W (ECU-F 100, 101), collected 21 July 2006 by M. Pepper and E. Twomey).
Etymology. The specific epithet is composed of the Latin adjective ‘ altus’, meaning ‘high’, and the Spanish adjective ‘ Amazónica ’, meaning ‘pertaining to the Amazon’. Combined to signify ‘upper-Amazonian’, referring to the species’ distribution, being known from the upper Río Huallaga and Ucayali drainages, both major headwaters of Río Amazonas.
Definition and diagnosis. A small species of Ameerega with an adult SVL of approximately 18–25 mm. Dorsal skin granular, black or brown, usually with white dorsolateral stripes extending from loreal region to groin. Yellow or orange spots present above groin, under axillae, and on shanks. White labial stripe present starting behind nares and ending at forelimb. Tadpole dark brown with large white spots lateral to mouth.
The combination of white dorsolateral stripes and a blue venter with black marbling distinguish A. altamazonica from the multitude of Ameerega species possessing one but not both of these characteristics. Ameerega yungicola and A. hahneli sensu stricto are the only other species of Ameerega possessing both these characters. Ameerega yungicola has maxillary teeth (teeth absent in A. altamazonica ), red (versus orange or yellow in A. altamazonica ) flash marks, and an advertisement call consisting of 4.5–5.0 notes per second with a dominant frequency of 3590-3719 Hz (versus 2–3 notes per second with a dominant frequency of 4300– 5140 Hz in A. altamazonica ). Ameerega hahneli sensu stricto is morphologically most similar to the new species but has an advertisement call consisting of 6–10 notes per second for several minutes. Its tadpole has enlarged and pointed (versus reduced and rounded in A. altamazonica ) marginal papillae on the posterior labium. Other species similar in appearance to A. altamazonica include A. boliviana , A. picta sensu stricto, A. petersi , A. pulchripectus , (all of which have yellowish or green dorsolateral stripes), and A. rubriventris (which has a reddish-orange venter instead of a blue venter in A. altamazonica ). The last mentioned species is the sister taxon to A. altamazonica , genetically distinct on the basis of 24 unambiguous nucleotide substitutions.
Measurements (in mm). The male holotype ( Figs. 1 View FIGURE 1 & 2 View FIGURE 2 ) has SVL 21.4; FL 10.4; FL 10.9; KK 20.0; FoL 10.2; HaL 10.7; HL 6.8; HW 6.1; BW 6.5; UEW 1.6; IOW 3.0; IND 2.9; TD 1.4; ED 1.6; DET 1.8; L1F 3.2; L2F 2.9; W3D 0.6; W3F 0.3. For paratypes from Tarapoto and Chazuta regions see Table 1 View TABLE 1 .
Description of type series. A small species of Ameerega , adults 17.4-24.5 mm, males slightly larger than females: 15 adult males 17.4–22.9 mm (mean 20.5 mm), six adult females 18.0– 24.5 mm (mean 22.1 mm). Sexual dimorphism is is exhibited only by males being slightly smaller and possessing vocal slits and a subgular vocal sac. Maxillary teeth absent; tongue gray, ovoid, attaching anteriorly on front one-quarter; choanae circular. Skin granular, especially on the dorsum and the dorsal surfaces of hindlimbs. Dorsal surfaces of forelimbs slightly granular, flanks and venter non-granular. In life, dorsal coloration of the head, back, and limbs ranges from black (as in holotype and topotypes) to copper-brown, thin white dorsolateral stripe usually present including holotype (one population lacks dorsolateral stripes, see below). Flanks black, some individuals have light blue spotting (spots absent in holotype). Venter smooth, dark to light blue with black marbling, yellow (as in holotype) or orange spots present under axillae and on medial face of tibia. Limbs light to dark brown on dorsal surfaces, ventral surfaces of forelimbs are light blue distally, yellow proximally. Underside of head pigmented as the venter but often darker (i.e. more black marbling), more evident in males. Iris dark brown with golden ring around pupil. All light and flash colors fade to white or gray in preservative.
Head widest at jaw articulations, slightly narrower than body in most individuals (head width at tympanum 95% of body width at axillae); head width 26–31% of SVL. Snout sloping laterally; bluntly rounded dorsally; truncate ventrally. Nares situated and directed posterolaterally to the tip of snout; nares visible from front and below, not so from above. Canthus rostralis sloped, slightly rounded; loreal region slightly concave (nearly vertical). Interorbital distance nearly same width of superior upper eyelid. Eye large and prominent, with a maximum diameter of 9.9% of the snout vent length; pupil rounded and horizontally elliptical. Tympanum circular, partially concealed posterodorsally, lacking tympanic annulus; tympanum width 41.5% of eye diameter. Supratympanic fold absent.
Hands relatively small, length 26% of SVL. Relative length of appressed fingers: III> IV ≈ II> I. Discs moderately expanded, disc on finger III 1.6 times width of finger below disc. A large, circular outer metacarpal tubercle is present on the median base of the palm; a smaller inner metacarpal tubercle present on base of finger I; one well developed and prominent subarticular tubercle on fingers I and II, two on fingers III and IV.
Hind limbs relatively small, femur 44% of SVL, tibia 48% of SVL. Relative lengths of appressed toes IV> III> V> II> I; first toe short, barely reaching bottom of subarticular tubercle on base of second toe, with unexpanded disc; toes II and III with barely expanded discs (much smaller than finger discs), and toe IV and V with discs expanded (disc 1.5 times broader than adjacent phalanx). Inner and a smaller outer metatarsal tubercle present, somewhat protuberant with rounded surfaces. One slightly protuberant subarticular tubercle present on toes I and II, two on toes III, IV, and V. Hands and feet lacking supernumerary tubercles, lateral fringes, and webbing. No basal webbing or toe fringes.
Remarks on geographic variation. Individuals from near Chazuta closely resemble the individuals from the type locality in both colors and pattern. The Pampa Hermosa population from the west bank of the Ucayali has larger groin spots that are bicolored (orange and yellow). There seems to be a north-south transition in the Huallaga valley from northern frogs having a black dorsum with well-defined dorsolateral stripes to southern populations having a copper-brown dorsum with reduced (and in some cases absent) dorsolateral stripes. The population from near Tocache best demonstrates this shift; this population is described as follows: dorsal skin more granular (compared to conspecific populations to the north), color metallic copper- or rust-brown, dorsolateral stripes absent, pale blue-white labial stripes extending from the corner of the mouth to axillae. Some individuals possess faint tan stripes outlining the snout, though these stripes do not extend posterior past the eyes. In some individuals a faint dorsolateral ridge can be noted separating the dorsum from the flanks. Venter and flank coloration is identical: sky-blue with black reticulation. Bright-orange spots present ventrally under axillae, dorsally over groin, and on medial surface of shanks ( Fig. 2 View FIGURE 2 , middle right and bottom right) Vocalizations. The advertisement call for A. altamazonica ( Fig. 3 View FIGURE 3 ) is a ‘retarded trill’ (following the definitions by Lötters et al. 2003). Notes are repeated at a rate of 1.3–2 notes per second, duration of individual notes ranges from 60–80 ms. Dominant frequency is modulated; notes start at 4100–4300 Hz and end at 4600–4680 Hz. This single-note advertisement call is given most frequently in the evening as males chorus. We also noted a second call type in A. altamazonica consisting of two notes in quick succession (within 10 ms of each other), repeated once every 3–5 seconds. Unlike in the advertisement call, these notes are not frequency-modulated, though the first note is typically lower in frequency than the second by 100–150 Hz. This two-note call appears to function as an aggressive or territorial call and is most frequently heard in the early morning.
Schlüter (1980) published spectrographs of A. altamazonica advertisement calls (as Phyllobates pictus ) from the Panguana region of central Peru, which had notes repeated at a rate of twice per second and were frequency modulated. Morales (1992) published a spectrograph of a call from a frog he referred to as Dendrobates sp. 2, which was from Tingo Maria, and has many similarities with the A. altamazonica advertisement call (i.e. 2–3 notes per second, note duration 42 ms). Since he made no mention of ventral coloration of this species, we assume the venter was ‘unremarkable’ (i.e. blue), and since the call did not have notes of alternating frequency (as in A. rubriventris ), we suppose that Morales’ Dendrobates sp. 2 can be referred to A. altamazonica . Lötters et al. (1997) presented advertisement call data for A. hahneli sensu lato from Tarapoto (= A. altamazonica ), noting the similarity to those of A. rubriventris , although the latter species has a call with notes of alternating frequency.
The advertisement call of A. altamazonica is easily distinguished from that of A. hahneli sensu stricto, which consists of a train of notes repeated at a rate of 5–9 notes per second, each note with a duration of 11– 18 ms ( Schlüter 1980, Morales 1992, Haddad and Martins 1994, De La Riva et al. 1996, Köhler and Lötters 1999). Dominant frequency ranges from 2700–7000 Hz and is not frequency-modulated. Ameerega yungicola , has an advertisement call resembling that of A. hahneli but differs in that it has a lower dominant frequency (3590–3719 Hz), slower repetition rate (4.5-5 notes/second), and longer note duration (31-34 ms) ( Lötters et al. 2005). The call of A. altamazonica is similar to the call of A. rubriventris , except the latter species has notes of alternating frequency whereas the former does not.
Tadpole (measurements in mm). A stage 30 tadpole (ECU-F 102) was chosen for the description ( Fig. 4 View FIGURE 4 ). It was free-living but species identity was confirmed by sequencing the 16S and cytochrome b gene regions. Total length 19.6, body length 7.2, maximum width 3.3, depth 2.8. Snout rounded when viewed from above; body ovoid and elongate in dorsal view. Eyes black, dorsal but angled laterally, pupils white in preservative. Nares not forming tube, situated half-way between eye and tip of snout, directed dorsally. Spiracle sinistral; vent dextral. Ventral tail fin begins at tail base, dorsal tail fin begins just posterior to plane of vent opening, deepest depth 1.4 measured 1/3 towards distal end. Caudal musculature deepest at tail base, musculature depth 0.77.
The mouth is directed anteroventrally. Oral disc emarginate, anterior and posterior labia forming flaps free from body wall. Marginal papillae absent on anterior labium, present in one complete row on posterior labium. Papillae white, rounded; submarginal papillae absent. Jaw sheaths medium in longitudinal width, finely serrate. Anterior jaw sheath has a medial indentation with reduced serration, posterior jaw sheath Vshaped and has serration throughout. Lateral processes long, extending well past lower jaw. Labial tooth row formula is 2(2)/3. A-1 complete, A-2 with medial gap, same width as A-1. P-1, P-2, and P-3 complete; P-1 and P-2 equal width, P-3 shorter. In preservative, the head appears dark gray due to subdermal pigmentation. Dermal pigmentation on dorsum is uniform translucent white with small black melanophores. Ventral coloration is translucent gray; under a dissecting microscope the ventral skin appears white with large black melanophores concentrated around anterior half of body and over intestinal coils. Tail musculature white, fins white, melanophores are present in small, irregular clusters along length of tail. Life color was dark gray or black with two distinct white spots lateral to the mouth. While these spots are also present in other species (such as the sympatric A. bassleri ), in A. altamazonica the spots extend back almost to the eye; in other species the spots extend back only to the level of the nares. Two additional stage 30 A. altamazonica tadpoles (ECU-F 103, 104) were examined and agree with this description.
Our examinations of A. hahneli tadpoles note the following differences from A. altamazonica : (1) dorsal and ventral skin (in preservative) uniform black with white marbling on rump and snout (versus translucent skin with black melanophores in A. altamazonica ), (2) lateral edges of anterior labium not forming free-flap (versus flap distinctly free from body wall in A. altamazonica ), (3) marginal papillae on posterior labium conspicuously large and somewhat pointed (versus reduced and rounded posterior marginal papillae in A. altamazonica ), (4) keratin on all three posterior labial tooth rows reduced or absent (versus present in A. altamazonica ), and (5) tail heavily mottled (versus white with irregular melanophore clusters in A. altamazonica ). Our examination of two A. hahneli tadpoles from Iquitos (ECU-F 105, 106) agrees well with the description by Haddad and Martins (1994) of an individual from Presidente Figueiredo, Brazil. The tadpole of A. altamazonica appears to be similar to that of A. rubriventris based on the sketches and description by Lötters et al. (1997). Mouthpart formulas are identical in the two species, but the tadpole shape is slightly different in that A. rubriventris larvae are more elongate and have a slightly upturned tail near the tip.
Distribution and natural history. Ameerega altamazonica is distributed throughout the east-Andean versant and surrounding lowlands of central Peru at elevations of 150– 865 m. This species is distributed widely throughout Departamento San Martín, and known from scattered localities in the east-Andean versant of Departamentos Huánuco and Loreto ( Fig. 5 View FIGURE 5 ). Jackknife tests of BIOCLIM variable contributions in Maxent indicate precipitation seasonality (coefficient of variation of precipitation) contained the most information (i.e. resulted in the highest gain increase when used alone) used to generate the niche model of A. altamazonica . In A. hahneli , jackknife tests of BIOCLIM variables show that temperature seasonality contained the most information used in generating the niche model.
Ameerega altamazonica View in CoL is currently not known from the east-Andean versant of Pasco and Junín, though this is probably attributable to sampling deficit and not their distributional limit, as dendrobatid sampling has been poor in central Junín and Pasco. Toothless specimens referred to as Phyllobates pictus View in CoL by Silverstone (1976) from Chanchamayo (Departamento Junín) and Luisiana (Departamento Cusco), Peru, likely represent A. altamazonica View in CoL , being from the highlands of the east-Andean versant. A single individual included in our phylogeny from Ivochote, Cusco (labeled A. sp. aff. ‘hahneli’), likely represents a new species, being both genetically and geographically distant from the clade containing A. rubriventris View in CoL and A. altamazonica View in CoL . Ameerega altamazonica View in CoL appears to be a species associated with mountains, and although it does occur in lowland habitats, these localities are relatively close to the east-Andean versant (<35 km). This is in contrast to A. hahneli View in CoL sensu stricto, which occupies a more Amazonian distribution, occurring throughout Amazonian Peru, Bolivia, Colombia, and east into Brazil and French Guiana (Haddad and Martins 1994, Lescure 2000, Lötters et al. 2005, 2007, Grant et al. 2006). We are unaware of any populations of A. hahneli View in CoL sensu stricto that occur in montane habitats.
Ameerega altamazonica View in CoL is most common in disturbed habitats, especially near small creeks or along roads. This species is less common in secondary forest and rare in primary forest. The activity patterns appear to be distinctly crepuscular, being most easily found in the early morning (6–8 h) or evening (16–18 h), when males are calling vigorously. Males typically call from the leaf litter, but some individuals were also found calling from clearly exposed positions in leaves approximately 0.5 m above ground level. Clutches of eggs have not been found in the field, but in captivity this species deposits eggs in leaf litter or on plant leaves near the ground. Clutches typically contain 14– 22 eggs but can contain up to 26 (Mark Pepper pers. comm.). One uncollected male was observed transporting twelve tadpoles on 11 March 2006 in the type locality. Nearby water sources were limited to a small creek which for most of the year is not flowing and consists of a series of small lentic pools. We have found free-living A. altamazonica View in CoL tadpoles in roadside ditches along the Tarapoto-Chazuta road, co-occurring with tadpoles of A. bassleri View in CoL , A. trivittata View in CoL , and Phyllomedusa tomopterna .
Ameerega altamazonica may serve as a (Batesian?) mimetic model for two species of Allobates with which it occurs sympatrically. Though we have no toxin information data on A. altamazonica , we suspect that it is ‘mildly toxic’ like other species of semi-aposematic Ameerega (see Summers and Clough 2001). In some areas of northern San Martín, A. altamazonica is sympatric with Allobates femoralis sensu lato, a complex of weakly- or non-toxic species which (here) share a similar pattern of a black dorsum and white dorsolateral stripes. In the central Huallaga valley, A. altamazonica is sympatric with an undescribed species in the Allobates femoralis complex (identifiable by a distinct advertisement call), which has a copper-brown dorsum and closely resembles the local A. altamazonica . Ameerega altamazonica may at the same time be a Müllerian mimic with the sympatric A. hahneli sensu stricto. Also, the possibility exists that a black dorsum with white dorsolateral stripes is an ancestral state in Ameerega and does not have any mimetic function in these two species.
Conservation status. Following the IUCN Red List criteria (IUCN 2001), we suggest A. altamazonica be listed as Least Concern (LC) under the following criteria: (1) we estimate its area of occupancy at 48,300 km 2, and part of this range lies within two National Parks (Río Abiseo and Cordillera Azul), (2) it occurs extensively throughout suitable habitat and thrives even in disturbed areas, (3) population sizes are assumed to be large since it is common in several habitat types, (4) populations do not appear to be declining, and (5) demand for the pet trade is presumed to be low.
TABLE 1. Measurements (in mm) of Ameerega altamazonica from type locality and Chazuta. Averages (with standard deviation) were calculated from the type series plus 6 additional undeposited specimens from Tocache (MUSM 24939 – 24944).
| Character MUSM 26934 SVL 20.3 | MUSM 26935 20.9 | MUSM 26936 24.5 | MUSM 26937 21.4 | MUSM 26938 20.2 | MUSM 26939 21.0 | Average (N = 23) 20.5 ± 2.3 |
|---|---|---|---|---|---|---|
| FL 10.5 | 9.9 | 11.1 | 10.4 | 9.5 | 9.9 | 9.2 ± 1.1 |
| TL 11.4 KK 20.5 | 10.9 20.0 | 11.7 21.6 | 10.9 20.0 | 10.6 19.8 | 10.2 9.3 | 10.0 ± 1.0 18.2 ± 2.8 |
| FoL 9.1 | 10.0 | 20.8 | 10.2 | 9.8 | 9.2 | 9.4 ± 2.8 |
| HaL 6.1 HL 6.2 | 5.9 7.1 | 6.5 7.9 | 5.7 6.8 | 5.9 7.8 | 5.5 6.4 | 5.4 ± 0.7 6.5 ± 0.8 |
| HW 5.9 | 6.3 | 6.9 | 6.1 | 6.3 | 6.2 | 6.0 ± 0.6 |
| BW 6.6 UEW 1.6 | 6.4 1.6 | 6.9 2.1 | 6.5 1.6 | 7.3 1.7 | 7.0 1.7 | 6.2 ± 0.9 2.8 ± 0.5 |
| IOW 3.2 | 3.3 | 3.2 | 3.0 | 2.7 | 2.9 | 2.6 ± 0.4 |
| IND 2.9 TD 1.3 | 2.9 1.3 | 3.2 1.4 | 2.9 1.4 | 3.0 1.4 | 2.9 1.1 | 2.8 ± 0.2 1.0 ± 0.2 |
| ED 1.9 | 1.6 | 1.7 | 1.6 | 1.4 | 1.6 | 2.4 ± 0.4 |
| DET 0.6 L1F 3.5 | 1.1 3.5 | 1.2 3.6 | 0.8 3.2 | 0.9 3.5 | 1.0 3.5 | 0.7 ± 0.2 3.3 ± 0.3 |
| L2F 4.0 | 3.2 | 3.2 | 2.9 | 3.2 | 3.2 | 3.1 ± 0.3 |
| W3D 0.6 W3F 0.3 | 0.5 0.3 | 0.6 0.3 | 0.6 0.3 | 0.5 0.3 | 0.5 0.3 | 0.5 ± 0.1 0.3 ± 0.1 |
| SEX M | M | F | M | M | M |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.