Agriosphodrus dohrni ( Signoret 1862 )
|
publication ID |
https://doi.org/ 10.5281/zenodo.193604 |
|
DOI |
https://doi.org/10.5281/zenodo.6207653 |
|
persistent identifier |
https://treatment.plazi.org/id/4B2F87FC-FFE0-FF8B-ABDE-BFA3D5770C11 |
|
treatment provided by |
Plazi |
|
scientific name |
Agriosphodrus dohrni ( Signoret 1862 ) |
| status |
|
Agriosphodrus dohrni ( Signoret 1862) View in CoL
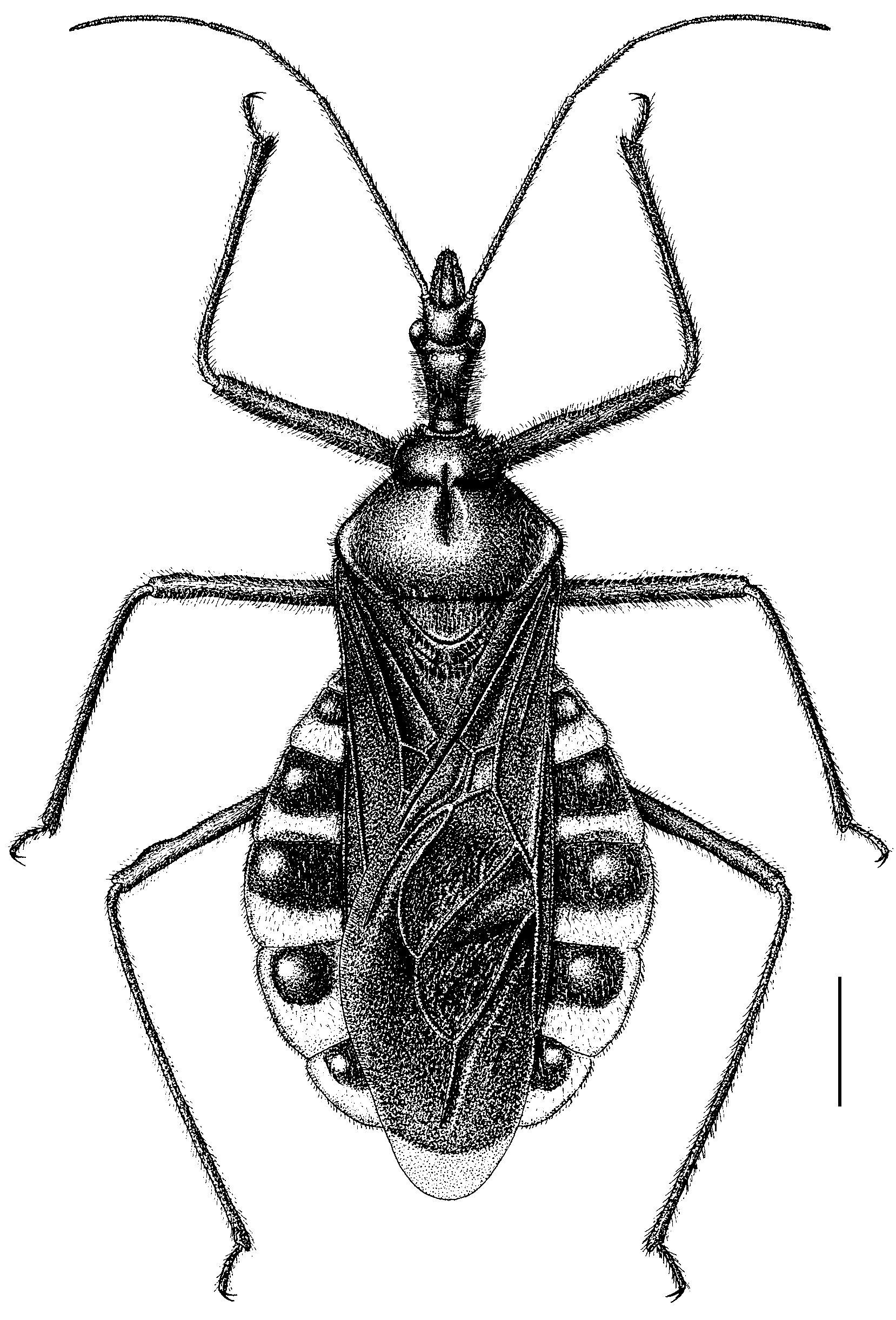
( Figs. 1–44 View FIGURE 1 View FIGURES 2 – 11 View FIGURES 12 – 19 View FIGURES 20 – 27 View FIGURES 28 – 35 View FIGURES 36 – 44 )
Redescription. Coloration: Body brightly black. Spots on external sides of ocelli, apical half of second to seventh and external margin of fifth to seventh connexival segments pale to dark yellow ( Fig. 1 View FIGURE 1 ); eyes, second and third segments of rostrum brown to black; central portion of seventh abdominal sternum of males and apical portion of abdomen of females red; apical portions of meso- and metapleura, lateral portion of each abdominal sternum with one whitish wax spot on each side; color of coxae diverse, some with fore and mid coxae red or yellowish, and some with fore coxae red or yellowish only.
Structure: Body densely clothed with long black erect setae (except rostrum, antennae, wings, and connexivum); first and second antennal segments with short setae, setae on third and fourth shorter; corium with short bent setae. Head long, slightly shorter than or subequal to the length of pronotum; rostrum long, extending to the posterior margin of anterior coxae ( Fig. 2 View FIGURES 2 – 11 ); ocelli widely separated; collar processes developed; anterior pronotal lobe rounded, posterior-central portion distinctly concave; posterior pronotal lobe shallowly concave in middle, posterior lateral pronotal angle rounded, posterior margin nearly straight; scutellum concave in the central portion; connexival segment significantly expanded, dorsal surface of segments 3–7 protuberant; membrane extending beyond tip of abdomen ( Fig. 1 View FIGURE 1 ). Pygophore oblong, ventral surface clothed with long setae, median process with a sharp process on each side ( Figs. 3, 4 View FIGURES 2 – 11 ); paramere clubbed, bent, subapical portion slightly thickened, apically rounded, with many long setae of different lengths ( Figs. 5, 6 View FIGURES 2 – 11 ); basal plate thin and curving, basal plate bridge thin, basal plate prolongation short, and wide ( Figs. 7–9 View FIGURES 2 – 11 ); phallotheca sclerotized dorsally and almost reaching to tip of phallosoma in resting condition; struts subequal to half of phallosoma in resting condition ( Fig. 10 View FIGURES 2 – 11 ); endosoma with many larger triangular spines and many small processes ( Figs. 8, 11 View FIGURES 2 – 11 ).
Measurements [3 (n=12) / Ƥ (n=11), in mm]: Body length 19.95–20.50 / 21.00–25.20; abdomen width 7.88–9.8 / 8.6–10.7. Length head 3.89–4.15 / 4.28–4.52; length anteocular portion 1.47–1.65 / 1.68–1.89; length postocular portion 1.52–1.73 /1.79–1.89; interocellar space 0.53–0.58 / 0.63–0.71; length synthlipsis 0.84–0.95 / 0.95–1.00; length antennal segments I–IV= 4.41–4.88 / 4.99–5.41, 1.63–1.84 / 1.84–2.10, 1.21– 1.26 / 1.23–1.26, 4.10 / 4.90;length rostral segments I–III=1.58–1.84 / 1.89, 2.68–3.05 / 3.26–3.36, 0.58–0.66 / 0.63–0.66; length anterior pronotal lobe 1.21–1.31 / 1.42–1.52; length posterior lobe 1.89–2.31 / 2.47–2.57; width thorax 3.47–4.73 / 5.12–5.41; length hemelytron 12.97–14.18 /15.50–17.00.
Material examined: 5 Ƥ, 8 3: China: Shaanxi, Xixiang, 2-V-1988, Wanzhi Cai leg; 1 Ƥ, 1 3: China: Guizhou, Guiyang, 23-V-1981, Chi-kun Yang leg; 1 Ƥ: China: Zhejiang, Jiangshan, Zhongwen Mao leg; 1 Ƥ: China: Duangdong, Nanling, VI-2000, Tao Zeng leg; 2 Ƥ, 2 3: China: Guizhou, Leigong Mountain, VI-2006, Ping Zhao leg.
Distribution: China (Anhui, Fujian, Gansu, Guizhou, Guangdong, Guangxi, Hainan, Henan, Hubei, Hunan, Jiangsu, Jiangxi, Shaanxi, Shanghai, Sichuan, Yunnan, Zhejiang), India, Japan, Vietnam.
Bionomics. Life history ( Figs.12–19 View FIGURES 12 – 19 ). Agriosphodrus dohrni is a univoltine assassin bug living on trees in Shaanxi Province, with 5 instars ( Figs. 14–18 View FIGURES 12 – 19 ). This species overwinters as fifth instars in cohorts beneath the bark or scars of trees like poplar. Overwintering 5th instars disperse from overwintering sites in early April of the next year. The peak of emergence occurs in mid to late April, and in early May the populations are mostly adults ( Fig. 19 View FIGURES 12 – 19 ). In the field, mating occurs in early to mid May. In the laboratory observations in Beijing, the adults mate about 13–28 d after emergence. The preoviposition period is about 15–20 d and the females lay eggs in mid May and June. The eggs ( Figs. 12, 13 View FIGURES 12 – 19 ) hatch in early June, and the nymphs develop from first to fifth instars ( Figs. 14–18 View FIGURES 12 – 19 ) from June to later October. The fifth instars start to overwinter in late October ( Tab. 1). There are significant differences among different stages in development duration ( Tab. 2).
, eggs;, 1st to 4th instars; ○, 5th instars; ●, overwintering 5th instars;, adults.
Stage Development duration (days)
minimum maximum average egg 16 22 17.33±2.64 1st instar 11 18 16.44±2.83 2nd instar 11 19 13.33±3.53 3rd instar 13 18 16.40±2.07 4th instar 19 22 21.10±3.40 5th instar 199 215 207.33±8.02 adult 76 86 82.33±5.50 Predatory behavior ( Figs. 20, 24 View FIGURES 20 – 27 ). Nymphs and adults of this reduviid mainly feed on the larvae of Lepidoptera in the field ( Fig. 20 View FIGURES 20 – 27 ). According to the sort and size of the prey, and the extent of starvation, the predatory process usually involves steps as: arousing and locating, approaching, paralyzing, sucking, releasing, and cleaning.
The assassin bugs get excited immediately when finding moving prey, and then most of them stretch the beak forward, lift the fore legs, sway the antennae forward and backward alternately, and move to the prey. Arousing and successful location is followed by a slow approach to the prey. This species has a slow gait and uses its long legs and rostrum to reach the prey.
After prey capture, the bug searches for a suitable site for stylet insertion and injects a toxic salivary secretion. When prey with firm tegument like yellow mealworms, the assassin bugs always choose intersegmental membrane or legs to stab. Prey (especially strong or big ones) always struggle fiercely when under attack, so a successful attack may involve several attempts by the bug. In all cases of successful inserting of stylets and injection of salivary toxins, the prey becomes totally paralyzed and dies in 20–30 sec. The prey is then sucked ( Figs. 20, 24 View FIGURES 20 – 27 ), in 30–60 min according to body size. If interrupted during this period, the assassin bugs will remove and drag the prey with their rostrum to a safer place. Sharing of the food is always observed in nymphal stages and adults ( Fig. 24 View FIGURES 20 – 27 ).
When satiated or the prey is used up, the assassin bugs will release the corpse of the prey, which is soft, wizened, and blackened, sometimes leaving only exoskeleton. Then they clean their antennae and rostrum several times with the apices of the fore legs.
Mating ( Fig. 21 View FIGURES 20 – 27 ). By laboratory rearing and field observation, we known that the mating process of Agriosphodrus dohrni essentially keeps to the same pattern, involving steps as: arousing, approaching, courting, clasping and riding, copulating, and cleaning.
Sexually mature males become excited when encountering females, swaying their antennae and lifting their fore legs. This arousing behavior lasts about 3–5 min. Then the male tries to excite the female, touching the body of the female by the apex of his anterior tibiae and using the antennae to touch one another. These actions will be repeated several times and last a few minutes.
After courting, the male will climb on the back of the female, and then insert his rostrum into the gap between the neck and pronotum of the female to fix his body. The male’s mid legs hold the mid coxae of the female, and his hind legs catch the apical portion of connexivum. This behavior is similar to riding, so we called this clasping and riding ( Fig. 21 View FIGURES 20 – 27 ). This lasts 0.5–1 day commonly. Finally, the male’s body inclines to one side and curves the tip of his abdomen to the female’s genitalia; at that point, the process comes to its true copulating time. This period is about 30–45 min.
The action of cleaning after mating is similar to that after predating; the positions include legs, antennae, and genitalia. After mating, the couple separates and then moves individually.
Oviposition ( Figs. 22, 23 View FIGURES 20 – 27 ). In the field, the gravid females of Agriosphodrus dohrni generally attach a cluster of a large number of eggs on the stems of trees. While placing the eggs, the females work from the margins to the center of the egg mass, gluing the eggs in vertical but oblique rows ( Figs. 22, 23 View FIGURES 20 – 27 ). Each egg is attached to the substratum as well as to the previously laid one, giving a special shape to the completed egg mass. The females cover such egg masses with copious secretions from their accessory glands and thus transform the egg masses into almost an ootheca. The whole egg mass contains 28– 65 eggs. The females will choose the location strictly to avoid the overcrowding of the newly hatched nymphs. When the population density is high, the females damage to other’s egg masses, especially in laboratory. The females perform parental care in a period of time after oviposition.
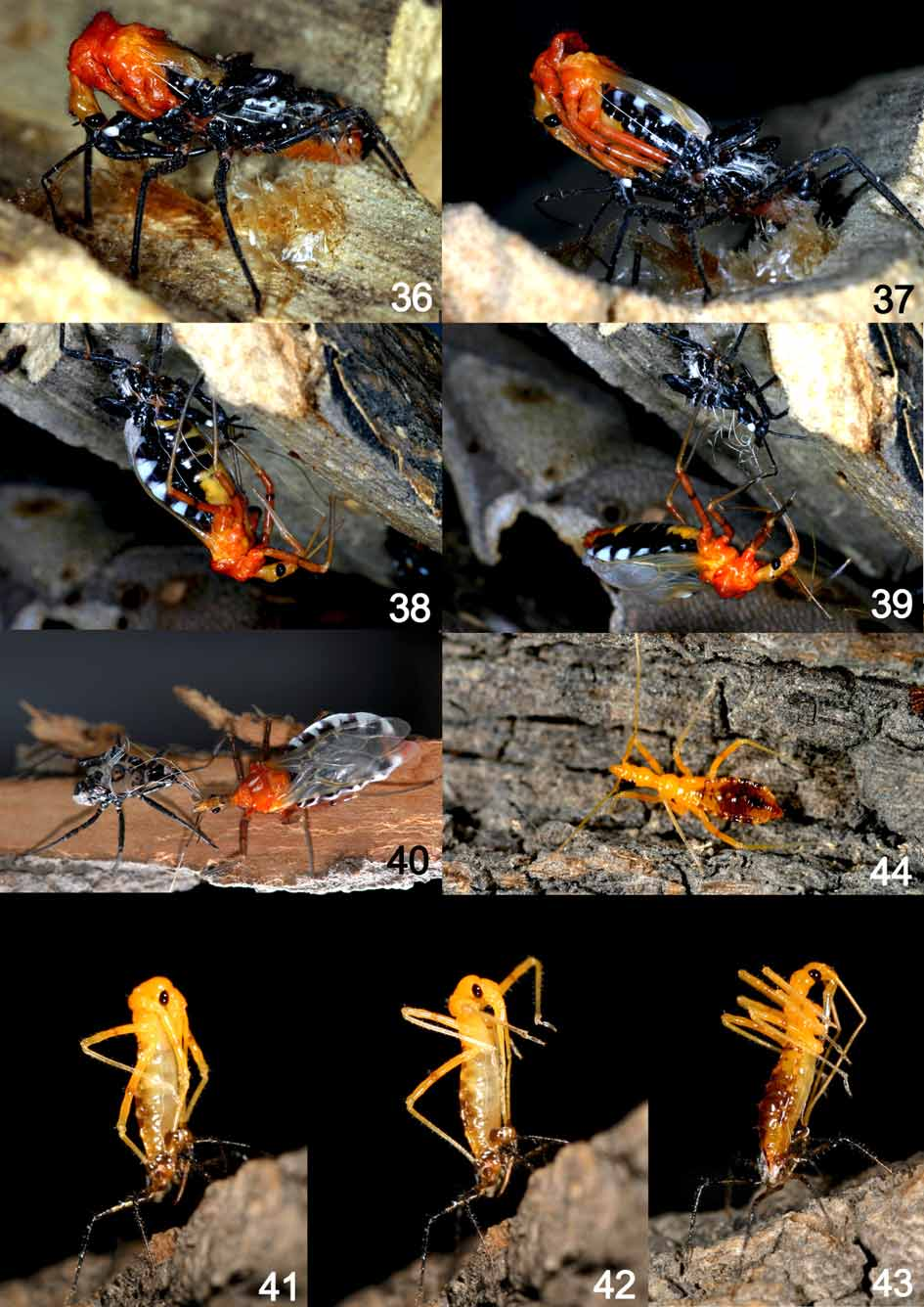
Hatching, molting, and emergence ( Figs. 28–44 View FIGURES 28 – 35 View FIGURES 36 – 44 ). The eggs often hatch from early of May to midJune. The following steps are involved in this process. Firstly head and pronotum of the nymph will expand out through removing the egg cap ( Figs. 28–30 View FIGURES 28 – 35 ). Then the fore legs will stretch out ( Fig. 31 View FIGURES 28 – 35 ), followed by the mid and the hind legs ( Figs. 32, 33 View FIGURES 28 – 35 ), and then the rostrum and the antennae ( Fig. 34 View FIGURES 28 – 35 ). The nymph will attempt to get out the chorion after stretching out its appendages, until it leaves the chorion completely ( Fig. 35 View FIGURES 28 – 35 ). The average time from opening the egg cap to leaving the chorion completely ( Figs. 28–35 View FIGURES 28 – 35 ) is about 10 minutes, and the total spending time of an egg mass hatching completely is about 30 min. The newly hatched nymphs are yellow all over; then they become darkened and hardened, and finally they start to be active in 20 h.
Before molting, the nymphs must become satiated and stop feeding, usually moving downwards. The first instars feed on small preys until they molt. At first the nymph makes a dorsal fissure on the pronotum extending from the front edge of the eyes to the first abdominal segment, then the head and the pronotum of 2nd instar will be stretched out from the fissure. After that, the appendages are stretched out in the order of mid legs, hind legs, fore legs, antennae, and rostrum. The exuviae is left at last. The molting process ( Figs. 41–44 View FIGURES 36 – 44 ) is measured as about 30 min, before a recovery process which takes about a few hours. After that the nymph will start to activate.
The process of emergence is similar to that of molting. The difference is that the vast major parts of the wings come directly from the longitudinal crevice of pronotum of the nymph exuvia, and the order for stretching out the appendages is mid legs, fore legs, hind legs, antennae, and rostrum. The remainder of the wings will be stretched out after all the appendages. Then the adult leaves the exuviae. The entire process ( Figs. 36–40 View FIGURES 36 – 44 ) takes about 50–60 min, and the recovery time is 12–22 h.
Colonization ( Figs. 25, 27 View FIGURES 20 – 27 ). Agriosphodrus dohrni performs significant colonization in its premature periods. The nymphs hatching from the same egg mass usually remain together during their entire premature period on the trees ( Figs. 25, 27 View FIGURES 20 – 27 ). They always attack and share the same prey. The adults are active and live alone. They are adept in flying and spread to other trees. The density is about 3–5 individuals per tree.
Cannibalism ( Fig. 26 View FIGURES 20 – 27 ). Nymphs of Agriosphodrus dohrni perform cannibalism. Hatching of a single egg mass is usually finished in a few hours and foraging activity is initiated 2 days after hatching. If newborns find other egg masses, they will gather around them until hatching take place. Consequently, it is sometimes observed that newborns obtain their first food by cannibalizing on hatching nymphs of other egg masses. When the population density is high, the molting and emerging individuals are often cannibalized by other nymphs and adults ( Fig. 26 View FIGURES 20 – 27 ). It was once believed that the cause of such phenomenon is the lack of food.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |