Affecauda salacia, Hall, Kathryn A. & Cribb, Thomas H., 2004
|
publication ID |
https://doi.org/ 10.5281/zenodo.158586 |
|
DOI |
https://doi.org/10.5281/zenodo.5688849 |
|
persistent identifier |
https://treatment.plazi.org/id/03822214-D119-FFE1-FED0-F95EFA80480D |
|
treatment provided by |
Plazi |
|
scientific name |
Affecauda salacia |
| status |
sp. nov. |
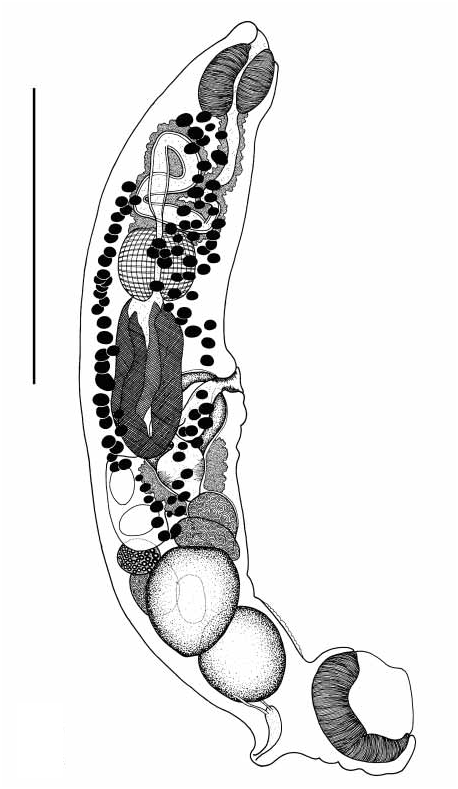
Affecauda salacia View in CoL n. sp. ( Figs 2–3 View FIGURE 2 View FIGURE 3 )
Typehost: Siganus corallinus (Valenciennes, 1835) , Siganidae ; ocellated, or bluespotted, spinefoot.
Site: intestine.
Typelocality: Lizard Island, Great Barrier Reef, Queensland, Australia (14°40'S 154°27'E, 1–9 Nov. 1998).
Prevalence: 4 of 5 (80.0%).
Intensity: 1
Deposition of specimens: holotype QM G222107, paratype QM G222108.
Etymology: This species name honours Salacia (Latin) , the wife of Neptune. In classical mythology, Salacia was the goddess of the sea.
Description: Based on 5 wholemount preparation of 4 mature and 1 immature worms. Measurements in Table 1 View TABLE 1 . Body elongate, extremely slender for entire length, rounded at ends, pronounced curvature dorsoventrally ( Figures 2–3 View FIGURE 2 View FIGURE 3 ); pale creamyyellow in life. Tegument distinctly annulated in posterior half of body at the region of the testes. Ventral sucker longitudinally elongate, at posterior extremity of body, on short peduncle. Pharynx elongate, barrelshaped, subterminal, surrounds mouth. Oesophagus long, sigmoid, with single loop, posterior chamber developed, invested with glands along entire length; lining thick. Oesophageal bulb of similar size to pharynx, ovoid, discrete. Caeca of equal lengths, terminate posterior to level of gesnital aperture.
Testes 2, entire, ovoid, tandem to subdiagonal, contiguous, in posterior third of body. Vasa efferentia not observed. Cirrussac present, spherical, ventral to caeca, encased in muscular capsule contiguous with pars prostatica and anterior portion of seminal vesicle. Seminal vesicle external, bipartite, both portions globular; proximal portion larger than distal portion. Pars prostatica large, cylindrical, tapering anteriorly, thickly surrounded by prostate gland cells, which partially penetrate cirrussac. Ejaculatory duct short, simple, expanding distally; everted cirrus not observed. Genital atrium large, convoluted, cilialike lining sometimes observed. Genital pore irregularly elliptical, median, ventral to caeca.
Ovary ovoid, pretesticular, median, overlapping anterior testis; oviduct associated with swollen insemination chamber. Seminal receptacle canalicular, globular, sinistrodorsal to ovary. Ootype anterior to ovary. Mehlis’ gland not observed. Laurer’s canal proceeds posterodorsally, opening dorsal to posterior testis. Vitellarium follicular; follicles largely restricted to anterior half of body, extend from posterior margin of pharynx to anterior margin of ovary; dorsal, ventral and lateral fields confluent. Vitelline reservoir globular, ventral to ovary. Uterus entirely preovarian. Eggs tanned, unembryonated in utero; opercula not observed. Opening of uterus unspecialised, enters genital atrium sinistral to cirrussac.
Excretory vesicle saccular; lining filamentous; pore opens on small, but distinct dorsosubterminal papilla. Primary collecting ducts arise anterolaterally; 1 pair only. Lymphatic system not observed.
Comments. Affecauda salacia is distinguished from A. annulata primarily by the combination of the presence of a loop in the oesophagus, contiguous testes and pretesticular ovary. By comparison, the oesophagus of A. annulata is sigmoid, the testes are not contiguous, and are separated by the ovary. Membership of A. salacia within the genus, however, is supported by features common to both A. salacia and A. annulata : the elongate and slender body shape; the position of the ventral sucker on a peduncle; and the position of the genital pore anterior to the termination of the caeca.
Specimens of A. salacia are morphologically similar to A. rugosa ; both species possess tandem, contiguous testes and a pretesticular ovary. The 2 species, however, may be differentiated by the tegumental annulations, the relative proportions of the oesophageal bulb and caeca, the shape of the seminal vesicle, and the length and position of Laurer’s canal. The annulation of the tegument of A. salacia is restricted to a small patch on the posterior half of the body, ventral to the testes. In contrast, the tegumental annulation of A. rugosa is evident along the entire length of the body. Further, the oesophageal bulb of A. salacia is proportionally larger than that of A. rugosa , and the caeca are conspicuously shorter, occupying approximately 1/6 of the body length; the caeca of A. rugosa occupy approximately 1/4 of the total body length. The seminal vesicle of A. salacia is bipartite, in contrast to the unipartite, globular seminal vesicle of A. rugosa . In specimens of A. salacia , Laurer’s canal is very long and proceeds posterodorsal from the ootype, opening dorsal to the posterior testis, whereas Laurer’s canal opens immediately dorsal to the ovary and ootype in A. rugosa . The wider body shape, in combination with the above features, makes specimens of A. salacia clearly distinct from A. rugosa .
Specimens of A. salacia show clearly the development of the powerful retractor muscles associated with movement of the ventral sucker ( Figure 3 View FIGURE 3 ). Retraction of the sucker is controlled by musculature anchored to the dorsal wall of the body. Muscle bands were observed anchored to the ventral sucker at 3 points: anterior, anterolateral and posterior.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Digenea |
|
Order |
|
|
Family |
|
|
Genus |