Dendrorhynchus sinensis Yin & Zeng, 1985
|
publication ID |
https://doi.org/10.1080/00222930600833909 |
|
persistent identifier |
https://treatment.plazi.org/id/EF73845C-FF9A-2531-FEA5-FB1DECDDFE61 |
|
treatment provided by |
Felipe |
|
scientific name |
Dendrorhynchus sinensis Yin & Zeng, 1985 |
| status |
|
Dendrorhynchus sinensis Yin & Zeng, 1985 ; Gibson, 1990 Polydendrorhynchus papillaris Yin & Zeng, 1986, 1988
Specimens examined
Type specimens of Dendrorhynchus zhanjiangensis Yin & Zeng, 1984 . Yin and Zeng (1984) designated the specimens Zhanjiang-8403 (including a holotype and a paratype) as the type specimens of D. zhanjiangensis . Deposited slides of Zhanjiang-8403 consist of six slides (Head 1–6) of the cephalic region, one slide (Middle 11) of the foregut region, 11 slides (Middle 1–10 and Middle 12) of the intestinal region, six slides (Tail 1–6) of the hind body region and three slides (Proboscis 1–3) of the proboscis. A bottle labeled Zhanjiang-8403 contains a complete specimen, two body fragments and two proboscides. The proboscis that has its basal axis cut off (for sectioning) and has six primary branches (four lateral and two terminal) is clearly the one illustrated by Yin and Zeng (1984, Figure 4) as the holotype. The small body fragment (about 22 mm long and 2 mm wide) lacks both anterior and posterior ends, which are presumed to have been sectioned by Yin and Zeng, and should be part of the holotype. The larger fragment (about 35 mm long and 4 mm wide) of the anterior body region, which has had the dorsal body wall dissected and the proboscis removed, should not belong to the holotype. The intact specimen is about 50 mm long and 3.5 mm in maximum width. As no clues have been found in the paper of Yin and Zeng (1984) or in Yin’s notes, it is not possible to determine which specimen they designated as the paratype. The specimens in bottle Zhanjiang-8403 are now re-sorted. Holotypic material (including a body fragment and a proboscis) are deposited in a bottle labeled Zhanjiang-8403; the complete specimen, whose cephalic region was transversely sectioned, and the foregut region dissected by the present author, are now numbered as Zhanjiang- 8403-1 (including section slides and body fragments); the other specimens (including a proboscis and a body fragment) are preserved in a bottle labeled Zhanjiang-8403-2.
There are two related bottles of preserved specimens labeled Zhanjiang-8403A and Zhanjiang-8403B. Zhanjiang-8403A contains six specimens 30–115 mm long and 2.0– 4.5 mm wide, two of them have been transversely cut into two and three fragments respectively, the other four are complete. Zhanjiang-8403B contains two specimens. The larger one is about 42 mm long and 2.5 mm wide, its everted proboscis possessing 13 exposed primary branches (11 lateral and two terminal); the smaller one is about 35 mm long and 2.5 mm wide with its dorsal anterior region dissected and its proboscis possessing 10 exposed primary branches (eight lateral and two terminal). Because there is no evidence that the cephalic regions of these specimens were sectioned and none of them were illustrated by Yin and Zeng (1984), the specimens of Zhanjiang-8403A and Zhanjiang- 8403B should not be included with the type series of D. zhanjiangensis .
Holotype of Dendrorhynchus sinensis Yin & Zeng, 1985 . Yin and Zeng (1985) designated specimen Zhanjiang-8304 as the holotype of D. sinensis . Transverse sections of the holotype consist of 16 slides of the cephalic region (Head 1–16), 14 slides of the intestinal region (Middle 1–14), 11 slides of the caudal end (Tail 1–11), four slides of the proboscis axis (Proboscis trunk 1–4) and six slides of a proboscis branchlet (Proboscis branchlet 1–6). Preserved specimens in the bottle labeled Zhanjiang-8304 consist of two body fragments (one about 48 mm long and 3.5 mm wide, the other 20 mm long and 4 mm wide) and two proboscides. One of these, with its basal portion cut off, has a branching pattern identical to that illustrated by Yin and Zeng (1985, Figure 4). This proboscis and the two associated body fragments (dissected by the present author) and slides, thus, form the holotype designated by the original authors. The other proboscis, which has 15 primary branches (13 lateral and two terminal), has been transferred to another bottle labeled Zhanjiang-8304A. This is not part of the type series of D. sinensis , because it was not mentioned in the original description, and the holotype was apparently the only specimen described by Yin and Zeng (1985). The basal region of the proboscis of Zhanjiang-8304A was transversely sectioned by the present author.
Type specimens of Polydendrorhynchus papillaris Yin & Zeng, 1986 . Yin and Zeng (1986) designate a holotype and a paratype (Zhanjiang-8409) for P. papillaris . Transverse sections of Zhanjiang-8409 consist of 26 slides of the cephalic region (Head 1–26), five slides of the intestinal region (Middle 1–5), 15 slides of the caudal end (Tail 1–15) and six slides of the proboscis (Proboscis 1–6).
Preserved specimens in the bottle labeled Zhanjiang-8409 consist of eight body fragments sorted into two bundles. The bundle consisting of two fragments of the middle body region (one about 60 mm long 5 mm wide, the other 82 mm long 4 mm wide) and a proboscis (the basal region of the trunk has been cut off, the branching pattern is identical to that illustrated by Yin and Zeng (1986, Figure 4) forms the holotype. A piece of foregut region and a piece of proboscis axis were sectioned by the present author.
The other bundle of specimens consists of four body fragments, including the head and the caudal ends, and a proboscis. This specimen comprises the paratype and is now preserved separately as Zhanjiang-8409A. The head and intestinal regions and proboscis axis were sectioned by the present author.
Other specimens. Three specimens (NH1, NH2 and NH3) were all dug from littoral mud at Potou. NH1 after preservation is about 210 mm long and 8 mm in maximum width, and consists of transverse sections of the anterior body, intestinal and hind body regions, and body fragments. NH2 is about 336 mm long and 17 mm in maximum width after preservation and consists of sections of various body regions and unsectioned body fragments. NH3 consists of body fragments (fixed with 95% ethanol) and transverse sections of the initial region of the unbranched proboscis axis. One additional preserved specimen (NH4), collected from Xiashan Beach Park by Yin Zuofen in 1974, about 440 mm long and 8 mm in maximum width, consists of dissected body fragments.
Many other individuals collected from Naozhou Island and Potou were observed in vivo or dissected.
Habitat and behavior
All specimens of Yin and Zeng (1984, 1985, 1986) were collected from the shore of Xiashan Beach Park. The environment of the type locality has been drastically changed due to the rebuilding of the park during the last decade, and no worms have been found at this location during recent years. Professor Feng Yuai, one of the collectors of the type specimens, told me that she usually found the worms under stones on sandy-mud substrata between tides in the 1980s. The worms often hid their posterior region in the substrate and protruded their anterior end into water left on the sediment surface. Similar behavior was noted by the present author at Naozhou Island on August 27, 2003. The worm would rapidly retract its exposed anterior region into its burrow if approached. Another observer, Chen Daohai, noted that the nemertean was often found near inlets of freshwater at ebb tide, and that its anterior body region often made oscillating movements in the water.
The nemertean has been recorded from different sediment types, including sand ( Gibson 1990), mud (present observation) and sandy-mud (Feng Yuai, pers. commun.).
The swimming behavior of the worms on the Xiashan coast was noted by Professor Yin Zuofen. In her fieldwork notes dated 9 February 1981, she wrote ‘‘(it) makes snake-like sidestroke, very rapidly’’.
The worm tends to evert its proboscis when stimulated, for example, when handled. As in Gorgonorhynchus repens Dakin & Fordham, 1931 , if the worm is strongly stimulated its proboscis may be thrown off (see Dakin and Fordham 1936). This is one reason for none of the present specimens fixed for morphological studies retaining their proboscis in situ. The thread-like branchlets of a detached proboscis may retain their ability to move for many minutes. In G. repens , however, ‘‘the detached proboscis almost entirely loses its mobility; only for a few seconds do the finest tips introvert and evert, probably due to the action of muscles of the wall on fluid which has not yet leaked away’’ ( Dakin and Fordham, 1936). The everted proboscis is so sticky that attempts to separate it from attached objects are rarely successful.
Like Polybrachiorhynchus dayi Gibson, 1977 View in CoL from South Africa and Cerebratulus lacteus (Leidy 1851) View in CoL from the United States ( Gibson 1977; McDermott 2001), Dendrorhynchus zhanjiangensis is used as bait by fishermen. Fishermen from Zhanjiang commented that compared with polychaetes, this ‘‘sea worm’’ was rare but an excellent bait.
External features
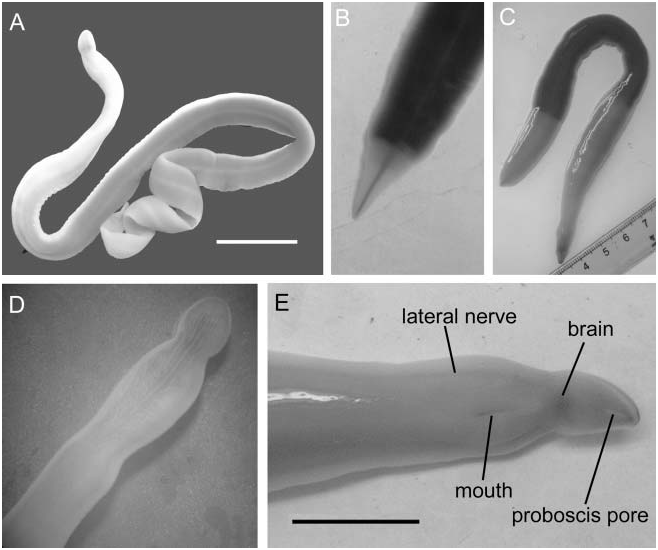
The living worm is ribbon-like, the posterior body (intestinal diverticular) region being more flattened than the anterior region ( Figure 1A View Figure 1 ). The maximum body width occurs in the anterior portion of diverticular region. Worms collected on 28 October 2002 are considerably larger than those recorded by Yin and Zeng (1984, 1985, 1986) and Gibson (1990). The largest living individual measured was 7.9 g in wet weight, and about 325 mm long and 16 mm in maximum width. The largest preserved specimen was 336 mm long and 17 mm wide. However, the shape of worms varies greatly in life, one example was noticed extending to a length of about 500 mm but was only 6 mm wide. Most worms are dark red, reddish-orange, or flesh-colored in the middle region of their body. The anterior region is paler than the succeeding regions ( Figure 1A View Figure 1 ), normally showing an orange color, sometimes bearing a slight tinge of greenish yellow on the dorsal surface. In some individuals, a paler posterior region is clearly marked off from the remaining body regions ( Figure 1B, C View Figure 1 ), which is supposed to be the result of regeneration. Through most of the body length, the white lateral margins are fin-like. The brain and lateral nerve cords can be seen in living worms, the latter appearing as a pair of red lines ( Figure 1E View Figure 1 ).
The head is marked off from the remainder of the body by a constriction ( Figure 1D, E View Figure 1 ). It possesses a pair of deep horizontal lateral cephalic slits. An elongate proboscis pore is situated ventrally near the anterior tip of the head ( Figure 1E View Figure 1 ). Eyes are absent. In some specimens a few longitudinal stripes can be traced on the dorsal surface of the cephalic region ( Figure 1D View Figure 1 ); these are irregularly arranged and paler than adjacent epidermis. A distinct rhynchodaeal groove, described for both Dendrorhynchus sinensis and Polydendrorhynchus papillaris by Yin and Zeng (1985, Figure 1 View Figure 1 ; 1986, Figure 1 View Figure 1 ), was not detected in either the present living worms or in sections of the holotypes of both species. It is likely that this groove was caused by retraction of the body. The mouth is slit-like and positioned immediately behind the brain ( Figure 1E View Figure 1 ). The length of the mouth varies according to the relaxed state of the body. In preserved specimens, it may be as long as the cephalic slits.
The posterior end of the body tapers to a slightly pointed tip ( Figure 1B, C View Figure 1 ). A dark ‘‘hindgut’’ can be seen in some living worms ( Figure 1B View Figure 1 ). There is no caudal cirrus.
Body wall, musculature and parenchyma
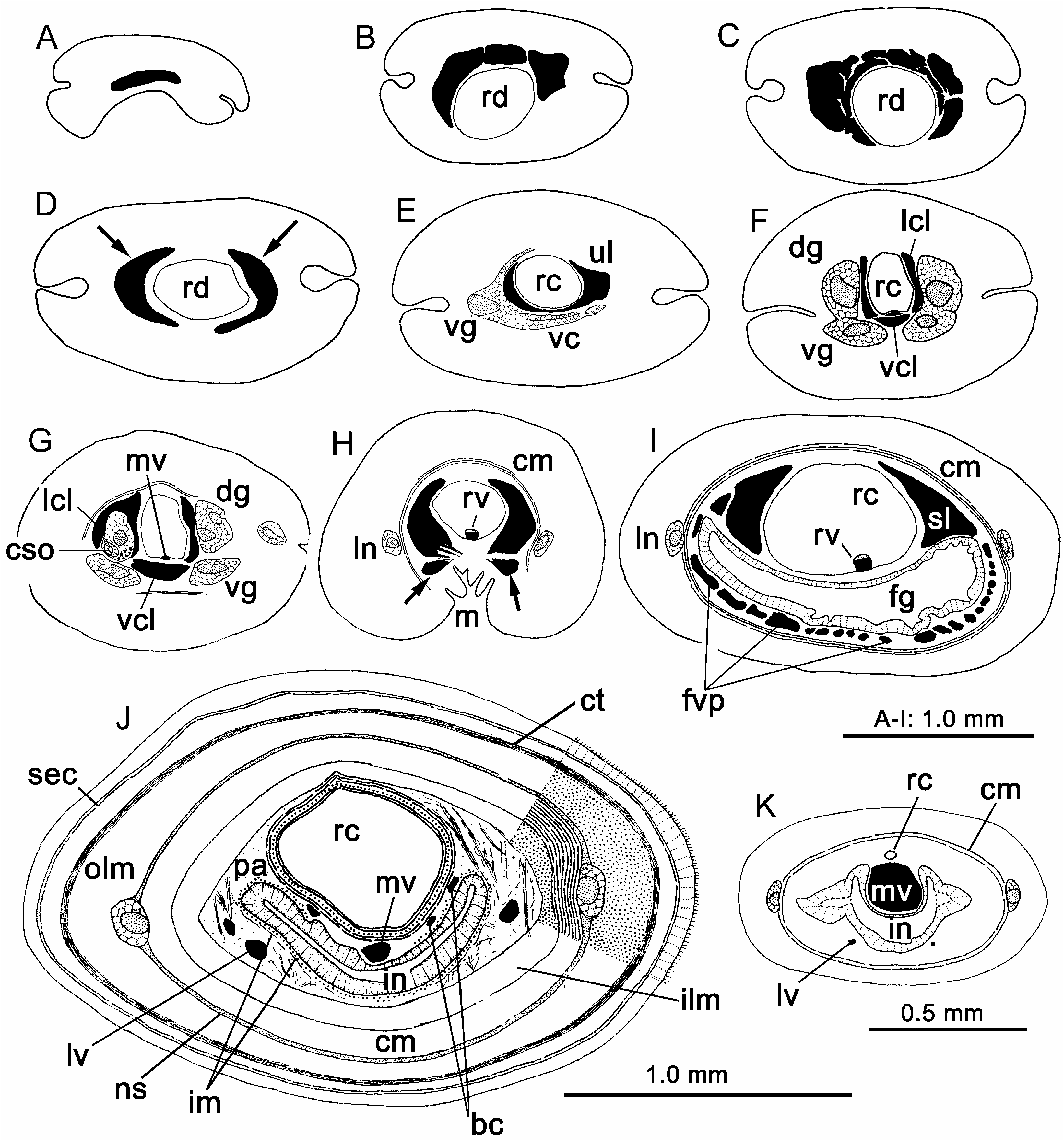
The epidermis contains numerous red-staining serous glands. A very thin zone of connective tissue can be detected between the epidermis and subepidermal circular muscle layer in some sections. The cutis consists of an outer subepidermal gland layer and an inner connective tissue stratum. The connective tissue layer is well developed in the foregut and anterior intestinal regions ( Figures 2A and 5J View Figure 5 ), but it disappears in the posterior body regions. Gibson (1990) gave a detailed description of the cutis. Specimens observed by the present author show no major differences from Gibson’s description. In the foregut region of the largest sectioned specimen (NH2), the maximum thickness of the eight main bodywall layers were respectively: epidermis,, 25 Mm; subepithelial circular muscle layer,, 15 Mm; subepidermal gland layer,, 150 Mm; connective tissue stratum,, 60 Mm; outer longitudinal muscle layer,, 440 Mm; neural sheath,, 15 Mm; circular muscle layer,, 180 Mm; inner longitudinal muscle layer,, 80 Mm.
Yin and Zeng (1984, 1986) and Gibson (1990) made no mention of the horizontal longitudinal muscle plate, and Yin and Zeng (1985, Table 1) indicated that the muscle plate was absent from Dendrorhynchus sinensis . Longitudinal muscle fibers, however, can be detected between the rhynchocoel and the foregut. These muscles are sometimes intermingled with the circular muscle fibers surrounding the dorsal wall of the foregut ( Figure 2B).
Parenchyma is extensive in the foregut region, the anterior intestinal region ( Figure 5J View Figure 5 ) and near the caudal end of the body, but it is only weakly formed in regions where the intestinal diverticula are well developed.
Dorsoventral muscles are present in the foregut and intestinal regions, but are only strongly developed in the intestinal region, where they pass between the intestinal diverticula.
Rhynchodaeum and rhynchocoel
The rhynchodaeum is lined by a ciliated epithelium and surrounded by a layer of circular muscles ( Figure 2C). The epithelium and cilia are mostly about 10–20 Mm and 15–20 Mm high respectively in the anterior rhynchodaeal region of the largest specimen sectioned (NH2). In the posterior region of the rhynchodaeum the epithelium is thinner and the cilia sparse.
The rhynchocoel extends for almost the full length of the body. In specimens relaxed with 7.5% MgCl 2 with their proboscis everted, the anterior region of the rhynchocoel is spacious and divided into a series of chambers by folds of its wall, with adjacent chambers connected by a narrow channel located near the dorsal rhynchocoel border ( Figure 3A, B View Figure 3 ). In a preserved specimen about 200 mm long and 15 mm in maximum width, some 30 chambers can be distinguished in the anterior region of the body. The most anterior chamber may be about 10 times the length of the remaining chambers. This condition of the rhynchocoel may be common in nemerteans with a branched proboscis. For example, the rhynchocoel of Gorgonorhynchus repens ‘‘is moniliform by the alternation of expanded and contracted sections’’ ( Dakin and Fordham 1936); the cross-sectional shape of the rhynchocoel of Gorgonorhynchus bermudensis Wheeler, 1940 , and Polybrachiorhynchus dayi varies considerably ( Gibson 1974, 1977). This type of arrangement of the rhynchocoel lumen may not always be clear to an observer. Specimens not anaesthetized but fixed directly with 95% ethanol are greatly contracted and a similar moniliform appearance could not be detected. This arrangement of rhynchocoel (as shown in Figure 3A, B View Figure 3 ) was not found in Yin and Zeng’s type specimens. However, in specimen NH4 collected from the type locality by Yin Zuofen in 1974, the moniliform arrangement of the rhynchocoel is distinct.
The rhynchocoel wall possesses an endothelium reaching 6 Mm thick in some places, a connective tissue stratum about 10 Mm in maximum thickness, and an outer circular and an inner longitudinal muscle layer ( Figure 2D). Yin and Zeng (1984, 1985, 1986) noted that the rhynchocoelic circular muscles of Dendrorhynchus zhanjiangensis , D. sinensis and Polydendrorhynchus papillaris were dorsally interwoven with the body wall musculature. Gibson (1990) indicated that in Hong Kong specimens of D. sinensis this muscle cross was not as strong as described by Yin and Zeng (1985). Present observations suggest that the development of this muscle cross varies greatly between different body regions. It is evident in some sections of the anterior intestinal region ( Figure 2E, F), but similar structures may also be detectable in some sections of the foregut region ( Figure 6A; also see Yin and Zeng, 1986, 1988, Figure 2). In addition, several muscle bands that dorsally link the rhynchocoel wall and the body wall muscles have been examined in sections of the anterior intestinal region ( Figure 6J).
Proboscis apparatus
The proboscis is a tree-like organ, consisting of a single main axis from which the primary branches arise laterally and alternately in the same plane. In contrast to the pinkish color of the proboscis in Gorgonorhynchus repens (see Dakin and Fordham 1936), the proboscis in the present species is white.
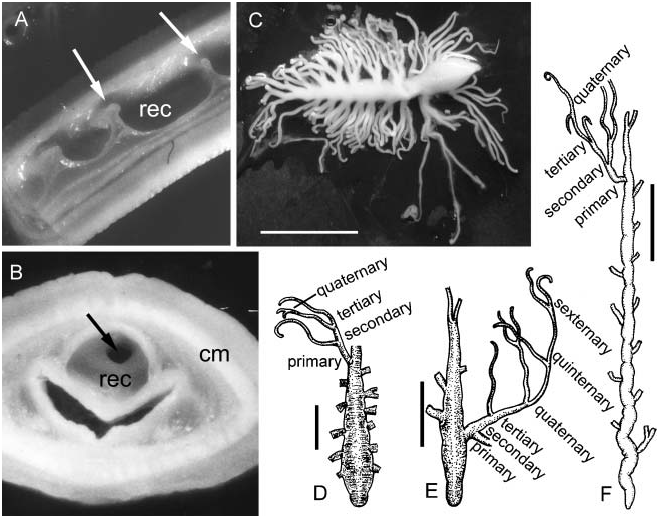
In the present specimens, the primary branches divide dichotomously two or three times ( Figure 3C View Figure 3 ) to yield successive branchlets, namely the secondary, tertiary and quaternary branchlets (see Gibson 1977). Yin and Zeng listed the absence of tertiary branchlets (between the secondary and terminal branchlets) in Dendrorhynchus sinensis as one of the characters for separating that species from D. zhanjiangensis (see Yin and Zeng 1984, Table 1), and indicated in a later paper that Polydendrorhynchus papillaris also lacked the tertiary branchlets ( Yin and Zeng 1986). However, a few branchlets in their illustrations for each species can be interpreted as tertiary branchlets ( Figure 3D, F View Figure 3 ; also see Yin and Zeng 1985, Figure 4; Yin and Zeng 1986, 1988, Figure 4). In addition, a primary branch in the holotype of D. zhanjiangensis apparently possesses quinternary and sexternary branchlets ( Figure 3E View Figure 3 ; see also Yin and Zeng 1984, Figure 4).
The number of branchlets that can be detected from each branching order varies considerably between individuals. Larger specimens tend to bear more branchlets at each branching level than smaller ones. The maximum number of primary branches is 20 (18 lateral and two terminal); the maximum number of terminal branchlets is 110 ( Figure 3C View Figure 3 ). Both numbers are much greater than recorded by Yin and Zeng (see Table I). However, it appears that in none of the specimens examined has the proboscis been either fully everted or retracted, and the complete branching pattern cannot be determined at present.
Yin and Zeng (1984, 1985, 1986) recorded that the main proboscis axis of D. zhanjiangensis , D. sinensis and P. papillari s is composed of eight distinct layers, an outer glandular epithelium, an outer connective tissue layer, an outer longitudinal muscle layer, a neural sheath, a circular muscle layer, an inner longitudinal muscle layer, an inner connective tissue layer and an endothelium. Gibson (1990) indicated that the Hong Kong specimens of D. sinensis possessed an additional inner circular muscle layer between the endothelium and inner connective tissue stratum. This muscle layer is distinct in the present specimens and in the holotype of D. zhanjiangensis ( Figure 2J) but was not detected in the type specimens of D. sinensis and P. papillari s, probably because the sections were not ideally stained.
Gibson (1990) illustrated two proboscis muscle crosses in D. sinensis , while Yin and Zeng (1984, 1985, 1986) described the proboscides of D. zhanjiangensis , D. sinensis and P. papillari s as possessing, respectively, one, one and no muscle crosses. The presence of two muscle crosses in all the sectioned proboscides can be confirmed by careful examination. In the holotypes of the two Dendrorhynchus species the weaker cross that was missed by Yin and Zeng is represented by a few muscle bundles leading from the circular muscle layer ( Figure 2H, I). In the holotype of P. papillaris , both muscle crosses are weakly developed ( Figure 2K, L).
In the initial part of the main axis, the proboscis is composed of an epithelium, an outer connective tissue layer, a longitudinal muscle layer separated into two portions by a neural sheath, an inner connective tissue layer and an endothelium. In this region, circular muscle layers and muscle crosses are absent, but distinct proboscis nerves can be seen in all the proboscides sectioned ( Figure 2G).
Digestive system
The mouth is an elongate slit opening ventrally a short distance behind the brain. The foregut possesses a subepithelial gland cell zone ( Figure 4A). In most places, the foregut epithelium and the subepithelial gland regions can only be distinguished by the nature of their glands, as in Polybrachiorhynchus dayi (see Gibson 1977). The epithelium and subepithelial gland cell zone together are up to 120 Mm deep in the ventral foregut wall, but weakly developed dorsally and normally less than half their ventral height.
As indicated by Gibson (1990), a circular muscle layer enclosing the foregut runs outside the subepithelial gland zone. On the dorsal foregut borders, the circular muscles are intermingled with longitudinal muscle fibers ( Figure 2B), which form a weakly developed muscle plate.
The structure of the intestine is similar to that of Polybrachiorhynchus dayi , consisting of two regions. A short anterior region forms a dorsoventrally compressed tube without lateral pouches. Posteriorly, as the body becomes broader and more compressed, the intestine begins to bear shallow lateral pouches. More posteriorly, the intestine is characterized by numerous deep diverticula and the main intestinal duct is reduced. This unusual distribution of lateral diverticula has been recorded in Dendrorhynchus zhanjiangensis by Yin and Zeng (1984) and P. dayi by Gibson (1977), but was not mentioned in the type specimens of D. sinensis and Polydendrorhynchus papillaris (see Yin and Zeng, 1985, 1986). Subdivision of the intestinal diverticula was not detected in the Zhanjiang worms, while the diverticula of P. dayi are subdivided in the posterior portion of the intestinal region ( Gibson, 1977).
As in P. dayi , the epithelium of the main intestinal tube and diverticula can be distinguished by their histological difference. The epithelium of the diverticula possesses more red-staining glands and more granular components than that of the main intestinal tube ( Figure 4B).
The intestinal tube is enclosed by an outer circular and an inner longitudinal muscle layer ( Figures 5J View Figure 5 and 6L). As having been recorded for P. dayi (see Gibson 1977), the circular muscles are derived from the dorsoventral muscles. The longitudinal muscle layer is strongly developed, and connections between this muscle layer and body wall musculature were not found. In some sections of the anterior intestinal region, the longitudinal splanchnic muscle layer may reach the thickness of body wall inner longitudinal muscle layer.
Blood system
The blood system is similar to that of Polybrachiorhynchus dayi . The cephalic blood supply, beginning as a transverse connective in front of the proboscis pore ( Figure 5A View Figure 5 ), consists of a series of inter-connected lacunae surrounding the dorsal and lateral margins of the rhynchodaeum ( Figure 5B, C View Figure 5 ). It forms two crescent-like lacunae near the proboscis insertion ( Figure 5D View Figure 5 ). In the anterior cerebral region, these two lacunae fuse ventrally to form a U-shaped lacuna ( Figure 5E View Figure 5 ). More posteriorly the U-shaped lacuna is ventrally divided by connective tissues and muscle fibers, from where a mid-dorsal blood vessel and a ventral cerebral lacuna emerge ( Figures 4D and 5F, G View Figure 5 ). The mid-dorsal blood vessel runs for a short distance in the rhynchocoel wall, and then forms the rhynchocoelic villus ( Figures 4H and 5H, I View Figure 5 ). At the rear of the cerebral region, the lateral cerebral lacuna expand to bathe the rear portion of the cerebral sense organs ( Figures 5G View Figure 5 and 6I). The ventral cerebral lacuna, namely the ‘‘esophageal blood vessel’’ (see Dakin and Fordham 1936; Yin and Zeng 1984, 1985, 1986), is inter-connected with the lateral lacuna in some places. This lacuna divides into two near the mouth ( Figure 5H View Figure 5 ). More posteriorly, blood lacunae are subdivided to form a foregut vascular plexus, which contains two spacious lacunae flanking the rhynchocoel ( Figure 5I View Figure 5 ). In the rear foregut region, these two lacunae become thicker-walled and continue posteriorly as the main lateral vessels. Throughout most of the intestinal region, the blood supply consists of a pair of lateral vessels and a middorsal vessel, linked by a series of transverse connectives ( Figure 5J View Figure 5 ). Near the anus, the mid-dorsal vessel is clearly expanded in some specimens ( Figure 5K View Figure 5 ). A distinct anal lacuna, linking all three longitudinal blood vessels, as indicated by Yin and Zeng (1984, 1985, 1986), was not detected in the present studies.
Gibson (1990) stated that ‘‘ Yin and Zeng (1984) distinguish between them principally in that compared with D. sinensis , D. zhanjiangensis exhibits a reduced proboscis branching, different dermal connective tissue organization and has no rhynchocoelic villus’’ (p 137). Furthermore, he states that Dendrorhynchus and Polybrachiorhynchus resemble each other in ‘‘…, foregut vascular plexus and rhynchocoelic villus (erroneously indicated as missing from Polybrachiorhynchus in Yin and Zeng’s 1985 table)’’ (p 138). Gibson (1990) evidently misunderstood Yin and Zeng’s term ‘‘mid-dorsal rhynchocoelic blood vessel’’ to mean rhynchocoelic villus. In Yin and Zeng’s (1985, Figure 3 View Figure 3 ) paper, a weakly stained structure situated close to the dorsal rhynchocoel wall was called a ‘‘mid-dorsal rhynchocoelic blood vessel’’ (also see Figure 2E) and, therefore, listed as present in D. sinensis ( Yin and Zeng 1984, Table 1; 1985, Table 1), but absent from D. zhanjiangensis ( Yin and Zeng 1984, Table 1) and from the genus Polybrachiorhynchus ( Yin and Zeng 1985, Table 1). The rhynchocoelic villus, termed ‘‘rhynchocoelic ridge’’ by Yin and Zeng, was reported present in D. sinensis , D. zhanjiangensis and P. papillaris by Yin and Zeng (1984, 1985, 1986). The so-called ‘‘mid-dorsal rhynchocoelic blood vessel’’ of Yin and Zeng is interpreted as a rhynchocoelic nerve in the present studies ( vide post).
The main longitudinal vessels are thick-walled and bear inner circular and outer longitudinal muscle fibers, but the longitudinal muscles are only well-developed in the middorsal vessel.
Excretory system
Although Yin and Zeng (1984) indicated that in Dendrorhynchus zhanjiangensis the excretory collecting tubules extended to the anterior intestinal region, this was not found in the present studies in the deposited slides of Zhanjiang-8403 (slides Middle 1–12), which represent the foregut region (slide Middle 11) and the anterior intestinal region (slides Middle 1–10 and 12). In fact, none of the deposited slides of the holotypes of D. sinensis , D. zhanjiangensis and Polydendrorhynchus papillaris consist of complete series of sections from the head to the anterior portion of the intestinal region.
In the sections of specimen Zhanjiang-8403-1, the excretory system emerges a short distance behind the mouth and extends posteriorly to the anterior border of the intestinal region. Most of the nephridial ducts spread alongside the ventral and lateral margins of the foregut ( Figure 2A) and are mostly in intimate contact with branches of the foregut vascular plexus. A pair of main longitudinal collecting ducts runs dorsolaterally at the outer margins of the foregut ( Figure 4C). These ducts may be branched and extend for about the anterior third of the full length of the excretory system. Each of the main collecting ducts posteriorly leads to an efferent canal, which enters the body wall and may run posteriorly for a short distance in the body wall musculature before discharging on the dorsolateral body surface ( Figure 2A). Flame cells (end organs) as illustrated by Dakin and Fordham (1936; plate V, Figure 22) in Gorgonorhynchus repens were not found in the present study.
Nervous system
The central nervous system is enclosed by connective tissue. Fibrous cores of the cerebral ganglia and lateral nerves are also enclosed by connective tissue (neurilemma). The fibrous core of the dorsal cerebral lobes, as described by Yin and Zeng (1984, 1985, 1986), is posteriorly forked into upper and lower branches ( Figure 5G View Figure 5 ). The lower branch posteriorly leads into the cerebral organ ( Figure 5G View Figure 5 ). In addition to the well-developed ventral cerebral commissure, another transverse connective between the ventral cerebral lobes, about 20 Mm thick, is present in the posterior portion of the cerebral region ( Figure 4D). At the front of the brain, from both dorsal and ventral ganglia numerous cephalic nerves emerge, with up to 30 or more on either side clustered along the lateral borders of the rhynchodaeum.
Yin and Zeng recorded that Dendrorhynchus zhanjiangensis and D. sinensis possessed one pair of neurochord cells ( Yin and Zeng 1984, 1985), while Polydendrorhynchus papillaris possessed two pairs ( Yin and Zeng 1986). The so-called ‘‘neurochord cells’’ illustrated by Yin and Zeng (1984; plate I, Figure 7) for D. zhanjiangensis , by Yin and Zeng (1985; plate I, Figure 3 View Figure 3 ) for D. sinensis , and by Yin and Zeng (1986, 1988; plate II, Figure 5 View Figure 5 , the upper one) for P. papillaris are in the present study interpreted as buccal nerves (foregut nerves) ( Figure 4D, F). These buccal nerves originate from the posterior portion of the ventral cerebral lobes, at first extending posteriorly in the ganglionic tissues ( Figure 4D) before emerging to run posteriorly below the rhynchocoel. After leaving the ganglionic tissues, the two buccal nerves are linked by a transverse connective ( Figure 4G). In the buccal region, each nerve is subdivided into a few small nerves ( Figure 4E), some of which extend posteriorly to the ventral margin of the foregut. The dorsal ‘‘neurochord cell’’ illustrated by Yin and Zeng (1986; plate II, Figure 5 View Figure 5 , the lower one) is enclosed by connective tissue and appears not to be a cell.
Many large cells, which may be 30 Mm or more in diameter and possess a distinct nucleus, are found around the fibrous cores of the dorsal and ventral cerebral lobes ( Figure 6B–E). The appearances, number and distribution of these cells are more similar to the Type III cells illustrated for heteronemerteans by Bürger (1895; plate 24, Figures 1–5 View Figure 1 , 21, 23, 25, 26) than the neurochord cells illustrated by him ( Bürger 1895; plate 24, Figures 3 View Figure 3 and 5 View Figure 5 ). In the holotype of P. papillaris , a structure which is much bigger than the Type III cells (about 40 Mm in diameter), is present in the ganglionic tissues of the posterior portion of each ventral cerebral lobe ( Figure 6F). A distinct nucleus cannot be distinguished in this structure and whether or not it represents a neurochord cell could not be determined. Neurochords were not found in the lateral nerves.
Several transverse connectives between the two lateral nerves were found in the anterior body region, but an anal commissure between the nerves was not traced.
The mid-dorsal nerve ( Figure 6A), though difficult to distinguish in some sections, is present in all specimens sectioned. A rhynchocoelic nerve, interpreted as a ‘‘mid-dorsal rhynchocoelic blood vessel’’ by Yin and Zeng (1985, Figure 3 View Figure 3 ), is present in all the specimens examined histologically. It occurs not only where the muscle cross between the rhynchocoel and body wall is well-developed ( Figure 2E, F), but also where this muscle crossing is wanting.
Near the inner borders of ventral cerebral ganglia, the ventral cerebral commissure sends a pair of distinct nerves. They run anteriorly for a short distance and then enter the proboscis through the proboscis insertion ( Figure 6K) to form the nervous supply of proboscis.
Sense organs
Frontal organs were not detected in Dendrorhynchus zhanjiangensis by Yin and Zeng (1984), and were not mentioned in the original description of Polydendrorhynchus papillaris (see Yin and Zeng 1986). Yin and Zeng (1985) stated that ‘‘the cephalic glands (of D. sinensis ) open to the outside of the body through the front organ at the dorsal part of the head’’, but did not describe the structure of the frontal organ. The organs, though small in size, were detected in a few of the specimens sectioned (Zhanjiang-8304, Zhanjiang-8403-1 and NH1) during the present study. They consist of three separate pits ( Figure 6G), about 65– 95 Mm in diameter. The arrangement of the frontal organs is thus similar to that of Polybrachiorhynchus dayi , but differs from the trifoliate structure occurring in Gorgonorhynchus (see Gibson 1974, 1977).
Horizontal cephalic slits form a pair of deep ciliated grooves extending along the lateral margins of the head. The outer and inner portions of the slits possess epithelium with differing staining features. The outer epithelium contains red-staining serous glands as found in the epidermis. Away from outer margins, the serous gland density decreases and by about the middle of the slits they have disappeared completely. In contrast, the inner epithelium of the slits possesses longer cilia and its basal region contains abundant nuclei ( Figure 6H), as in species of the genus Gorgonorhynchus (see Dakin and Fordham 1936; Gibson 1974). At about the middle of the cerebral region, each cephalic slit leads to a ciliated cerebral canal, which finally enters the cerebral sensory organ from the ventrolateral border of the dorsal cerebral ganglion ( Figure 5G View Figure 5 ).
The cerebral sensory organs are fused to the lower branch of the dorsal cerebral ganglia ( Figure 5G View Figure 5 ). The most posterior portion of each cerebral organ is bathed by the spacious lateral cerebral lacunae and consists of a cluster of weakly stained gland cells ( Figure 6I).
Eyes are absent.
Cephalic glands
There are weakly stained glands scattered between the muscle fibers in the cephalic region. Although Yin and Zeng (1985) stated that ‘‘the cephalic glands (of Dendrorhynchus sinensis ) open to the outside of the body through the front organ’’, aggregated cephalic glands that discharge via the frontal organs, as recorded in Polybrachiorhynchus dayi (see Gibson 1977), were not found.
Reproductive system
No gonads were found in any of the sections examined.
Geographic distribution
Dendrorhynchus zhanjiangensis has been reported from the coasts of Xiashan ( Yin and Zeng 1984, 1985, 1986, 1988), Potou and Naozhou (present study), Zhanjiang City, Guangdong Province; and from Starfish Bay, Hong Kong ( Gibson 1990).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Order |
|
|
Family |
|
|
Genus |
Dendrorhynchus sinensis Yin & Zeng, 1985
| Sun, Shichun 2006 |
Dendrorhynchus zhanjiangensis
| Yin & Zeng 1984 |
Dendrorhynchus zhanjiangensis
| Yin & Zeng 1984 |
Polybrachiorhynchus dayi
| Gibson 1977 |