Antechinus stuartii, Macleay, 1841
|
publication ID |
https://doi.org/10.5281/zenodo.6608102 |
|
DOI |
https://doi.org/10.5281/zenodo.6602809 |
|
persistent identifier |
https://treatment.plazi.org/id/EA7087C1-FF89-2465-FFC4-F7C30BCA03CD |
|
treatment provided by |
Felipe |
|
scientific name |
Antechinus stuartii |
| status |
|
36. View On
Brown Antechinus
Antechinus stuartii View in CoL
French: Antéchinus de Stuart / German: Stuart-BreitfuBbeutelmaus / Spanish: Antequino pardo
Other common names: Macleay’'s Marsupial Mouse, Stuart's Antechinus
Taxonomy. Antechinus stuartii Macleay, 1841, View in CoL
Manly (Spring Cove, Sydney Har bour) , New South Wales, Australia.
The confusion associated with the taxonomy of A. stuartii View in CoL began with its description in 1841. Throughout most of its relatively long history, A. stuartii View in CoL has been embedded in the synonymy of A. flavipes because in 1924 O. Thomas assigned it to his Phascologale flavipes var. typica from eastern Australia. W. J. Macleay in 1841 described the genus Antechinus View in CoL mistakenly convinced he was dealing with an antipodean insectivore ( Antechinus View in CoL means “hedgehog-equivalent”). Macleay had named the species after Captain J. Stuart, Assistant Colonial Surgeon, who provided him with a drawing and a written description of the animal, along with an erroneous incisor count (I 6/6). In fact, Macleay did not even personally examine a specimen in the process of describing it, although he later examined its skeleton. More than a century later when N. A. Wakefield and R. M. Warneke finally revised the genus in 1967, they emphasized the distinction of A. stuartii View in CoL from A. flavipes and reassigned the forms wunicolor (New South Wales), burrelli (north-eastern New South Wales), and adustus View in CoL (north-eastern Queensland) to the synonymy of A. stuartii View in CoL . S. Van Dyck and M.S. Crowther in 2000 raised adustus View in CoL to subspecies and then species status and designated subtropicus View in CoL (from south-eastern Queensland and north-eastern New South Wales) as a subspecies of stuartii View in CoL . Then, in 1988, it was discovered that A. stuartii View in CoL in New South Wales and Victoria was composed of two electrophoretically distinct forms that occurred sympatrically at Kioloa, south-eastern New South Wales. The northern form (A. stuartii View in CoL sensu stricto) was distributed from south-eastern Queensland to around Kioloa; the southern form (now A. agilis View in CoL ) was distributed throughout southern New South Wales and Victoria. Finally, the subspecies subtropicus View in CoL was later raised to full species status based on genetics and an asymmetric mating period with sympatric stuartii View in CoL in north-eastern New South Wales. Thus, what had long been known as the Brown Antechinus, with a distribution stretching from southern Victoria to north-eastern Queensland, was discovered to consist of four genetically distinct species of antechinuses: stuartu, agilis View in CoL , subtropicus View in CoL , and adustus View in CoL . Consequently, distribution of stuartii View in CoL was much reduced. Although similar in morphology, all these species show profound genetic differences. A. stuartii View in CoL is sister to A. subtropicus View in CoL , and these two species, with A. agilis View in CoL , form a well-supported clade to the exclusion of other antechinuses; A. adustusis in fact genetically more closely allied with a clade containing A. bellus View in CoL , A. leo View in CoL , A. flavipes , A. mysticus View in CoL , and A. argentus View in CoL . The most recent research has indicated that there is a genetic break in A. stuartii View in CoL in northern New South Wales that requires further investigation; southern and northern forms of A. stuartii View in CoL closely abut and may even be sympatric in some areas. There are doubtless a few more twists in the tale of A. stuartii View in CoL to come. Monotypic.
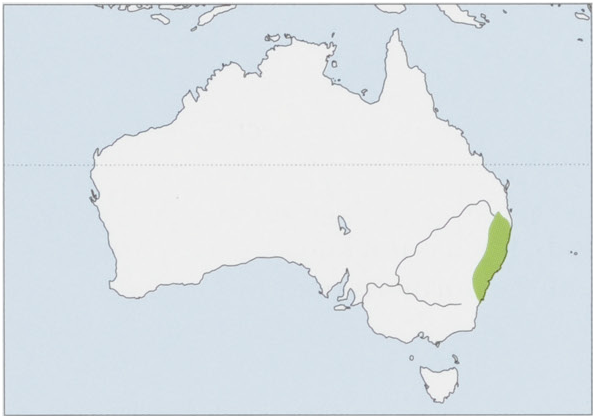
Distribution. E Australia, from the Main Range National Park in S Queensland to about Kioloa in SE New South Wales. View Figure
Descriptive notes. Head—body 8:1-14.1 cm (males) and 7:3-11.6 cm (females), tail 7-124 cm (males) and 6.5-11.6 cm (females); weight 24-71 g (males) and 16-40 g (females). There is marked sexual dimorphism for size. The most obvious character of external morphology in the Brown Antechinus is the general uniformity of its fur color, which dorsally is snuff brown (pale chocolate color) and ventrally a paler buffy brown. Many specimens show a slight warming of brown from mid-dorsum to rump, but this is most obvious only under bright illumination. Forefeet and hindfeet are drab, pale grayish-brown. The Brown Antechinus lacks distinct eye rings (although there can be pale dabs of “eyeliner” in northern specimens) and post-auricular patches, and tail is a uniformly drab gray-brown. In some specimens,tail tip is slightly darker than the tail base, but this dark brown cannot be confused with marked fuscousblack tail tip of the Yellow-footed Antechinus (A. flavipes ). The Brown Antechinus is most similar in terms of external coloring and skull morphology to the Subtropical Antechinus (A. subtropicus ) and, to a lesser degree, the Agile Antechinus (A. agilis ). The Brown Antechinus, particularly in the northern parts ofits distribution where it may co-occur with the Subtropical Antechinus, tends to be slightly more grayish on head and neck, merging to brownish on rump, whereas the Subtropical Antechinus from the same regions tends to be more uniformly brown from head to rump. Unfortunately, these trends are inconsistent and only recognizable with direct reference to comparative museum specimens; for all intents and purposes, the two species are indistinguishable in the field. The Agile Antechinus tends to be more grayish on head and rump, often with pale eye ring, rather than more uniformly brownish on head and rump, and the Brown Antechinus lacks an eye ring. In short, these differences in coloration and skull features between the Brown Antechinus and the Agile Antechinus and even more so between the Subtropical Antechinus and the Brown Antechinus are very subtle.
Habitat. A variety of habitat types, including tall woodland, wet and dry sclerophyll, cool temperate rainforest, logged dry sclerophyll, pine forest, swamp, dune-swale, subalpine woodland, and heathland.
Food and Feeding. The Brown Antechinus is an opportunistic insectivore and eats beetles, spiders, amphipods, and cockroaches. It hunts forits prey in trees and on the ground.
Breeding. Male Brown Antechinuses become increasingly aggressive as winter progresses; their staccato bursts of “chee” vocalizations are heard more frequently as the males sort out their relationships before the mating season (August in southern Australia and September in northern New South Wales). Local synchrony of breeding is triggered by a particular rate of increase in daylength after winter solstice. Both sexes nest communally until late May, after which they become more solitary leading up to mating. Copulation is prolonged, typically lasting c.6 hours, with some males mating repeatedly. Both sexes mate promiscuously, and each litter can have multiple fathers because females store sperm in specialized ducts. Males die at the conclusion of the mating period, which is characteristic of the genus. Female Brown Antechinuses may breed in a second season, but success of reproduction in subsequent years is very low. Females typically have eight teats, but number varies from six to ten teats, being constant for any given locality. Gestation lasts ¢.27 days, and sufficient young are usually born to occupy every teat. Although young are dragged awkwardly across the ground until they are c.5 weeks old, their survival rate is remarkably high. From an age of c.5 weeks, they are left in a spherical nest of dry plant material hidden in a hollow log or tree trunk while their mother hunts at night; after young are fully mobile, they may hunt alongside their mother. Any association between mother and offspring decreases shortly after weaning at c.90 days of age. One study found that natal nests were gradually abandoned, with mothers and young making individual forays in search of alternate nesting sites, before they left permanently; mothers often abandoned young at the natal nest at weaning, meaning that juveniles of both sexes leave the nest without being forced by their mother.
Activity patterns. Most hunting in the Brown Antechinus takes place at night, butit may be active during the day, particularly during winter when food is scarce. In winter, individuals may go into torpor for a few hours at a time, thus reducing energy requirements during this period of low prey availability.
Movements, Home range and Social organization. In one mark-recapture study, male Brown Antechinuses dispersed farther (maximum distance 1230 m for males and 270 m for females) and more often (71% in males and 11% in females) than females. Males tended to disperse farther if they had been raised in an area of low density; they were also more likely to immigrate into an area with a higher density of females than their natalsite. Death of the mother tended to disrupt normal home range establishment, with a higher probability of philopatry in sons and dispersal of some daughters. Some females emigrated after young were weaned; this also prompted dispersal of daughters. No evidence was found to suggest daughters with surviving philopatric mothers were more likely to survive to breed. It was suggested that male-biased dispersal resulted not only from costs of inbreeding but also partly due to benefits of finding a site with more mating opportunities.
Status and Conservation. Classified as Least Concern on The IUCN Red List. The Brown Antechinus appears to be abundant throughout much ofits distribution; densities are often high where ground coveris thick and logs are abundant. Within its distribution, the Brown Antechinus is one of the most readily caught species of antechinus.
Bibliography. Baker et al. (2012), Crowther (2002), Crowther & Braithwaite (2008), Dickman et al. (1988), Fisher (2005), Fox & Archer (1984), Gould (1854), Le Souef & Burrell (1926), Macleay (1841), Thomas (1888b, 1923b, 1924), Van Dyck (1997), Van Dyck & Crowther (2000), Wakefield & Warneke (1967).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Metatheria |
|
Order |
|
|
Family |
|
|
Genus |
Antechinus stuartii
| Russell A. Mittermeier & Don E. Wilson 2015 |
argentus
| Baker, Mutton & Hines 2013 |
A. mysticus
| Baker, Mutton & Van Dyck 2012 |
subtropicus
| Van Dyck & Crowther 2000 |
subtropicus
| Van Dyck & Crowther 2000 |
subtropicus
| Van Dyck & Crowther 2000 |
A. subtropicus
| Van Dyck & Crowther 2000 |
A. agilis
| Dickman et al. 1998 |
agilis
| Dickman et al. 1998 |
A. agilis
| Dickman et al. 1998 |
leo
| Van Dyck 1980 |
Antechinus stuartii
| Macleay 1841 |
A. stuartii
| Macleay 1841 |
A. stuartii
| Macleay 1841 |
Antechinus
| Macleay 1841 |
Antechinus
| Macleay 1841 |
stuartii
| Macleay 1841 |
stuartii
| Macleay 1841 |
stuartii
| Macleay 1841 |
stuartii
| Macleay 1841 |
stuartii
| Macleay 1841 |
stuartii
| Macleay 1841 |
stuartii
| Macleay 1841 |
stuartii
| Macleay 1841 |
stuartii
| Macleay 1841 |
stuartii
| Macleay 1841 |
stuartii
| Macleay 1841 |
A. flavipes
| Waterhouse 1838 |
flavipes
| Waterhouse 1838 |
flavipes
| Waterhouse 1838 |