Hymenicoides Kemp, 1917
|
publication ID |
https://doi.org/10.5281/zenodo.179195 |
|
DOI |
https://doi.org/10.5281/zenodo.6251670 |
|
persistent identifier |
https://treatment.plazi.org/id/BB10DE28-6C3F-2C6E-4485-FC3DFE6E2ED0 |
|
treatment provided by |
Plazi |
|
scientific name |
Hymenicoides Kemp, 1917 |
| status |
|
Hymenicoides Kemp, 1917 View in CoL
Hymenicoides— Kemp, 1917: 267; Lucas, 1980: 196; Ng & Chuang, 1996: 50 (part); Guinot & Richer de Forges, 1997: 460 (part); Guinot & Bouchard, 1998: 685.
Type species. Hymenicoides carteri Kemp, 1917 , by monotypy; gender of genus masculine.
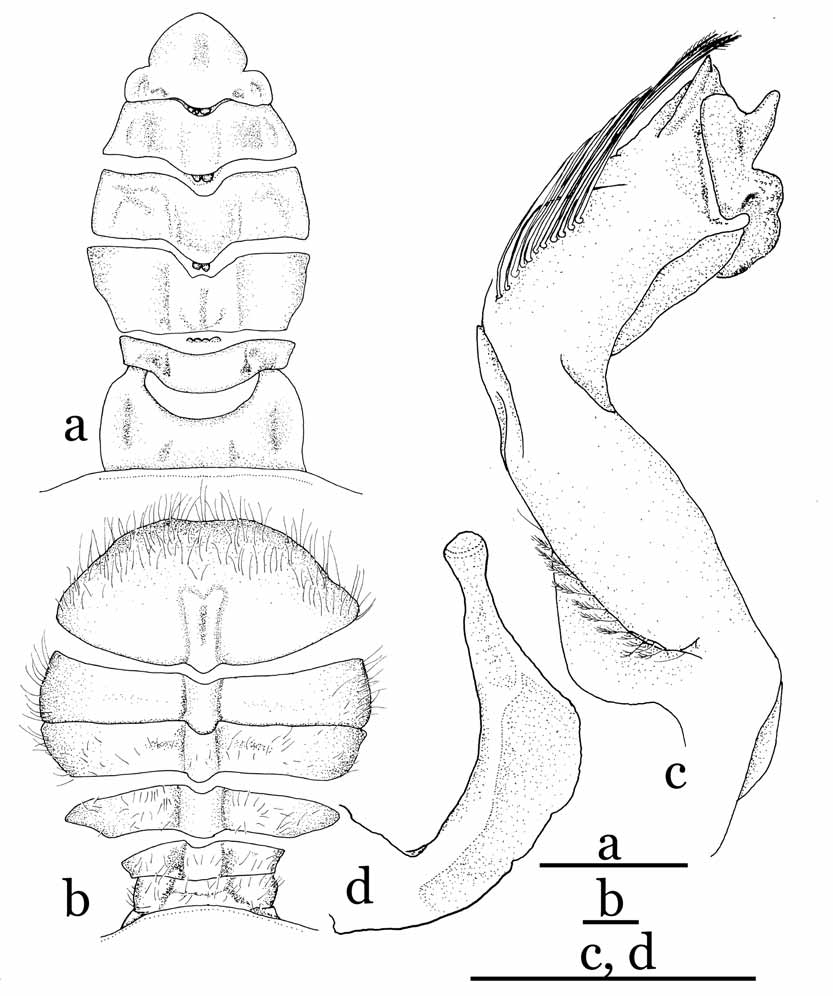
Diagnosis. Carapace oval, dorsal surface concave; grooves distinct; rostrum absent or very weak; antenna with base of basal article placed posterior-inner part of base of eye; eyes, antennae, antennules visible dorsally; third maxillipeds narrow, not covering more than three-quarters of mouth field when closed, merus rectangular, dactylus styliform, approximately twice length of propodus; male chelae relatively stout, manus with high, prominent, dorso-ventrally compressed tubercle on distal part of outer surface, tubercle absent in females. Female vulvae placed on imaginary line joining inner ends of sutures between sternites 5 and 6 on medially fused plate of thoracic sternum, vulva with basal mount. Male abdomen-pleotelson without fused segments, male pleotelson distinctly trilobed, inner surface thickened externally, forming socket for sternal button ( Fig. 1 View FIGURE 1 a). G1 stout, bent outwards medially; distal part with prominent distal inner processes, swollen distal outer angle, long, dorsal bursiform projections. Female abdomen with 6 distinctly demarcated segments, boundary between first and second segments ( H. carteri ) or between second and third segments ( H. robertsi ) movable; long, biramous pleopods on second to fifth segments, developed from distal outer end of inner surface of each segment.
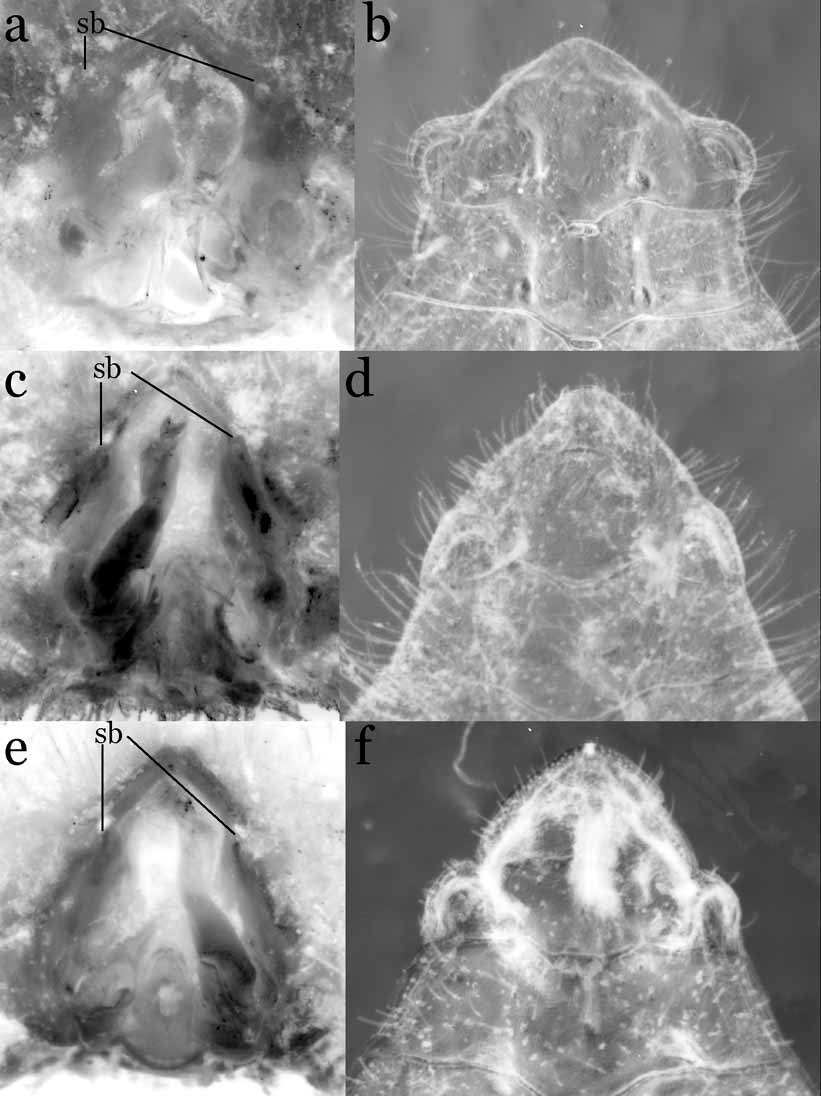
Remarks. The distinctive trilobate pleotelson of H. carteri has received some attention from taxonomists due to its unusual shape. Lucas (1980: 197) regarded that the lateral lobe of male pleotelson as “a cavity to receive the apice of the first pleopod”. Guinot & Richer de Forges (1997: 470) suggested that the lateral lobe of the pleotelson may be derived from the inserted plate (see Guinot & Richer de Forges, 1997: Fig. 6 View FIGURE 6 , for mobile intercalated platelet of Odiomaris pilosus ). Guinot & Bouchard (1998: 685) subsequently hypothesized that the abdominal socket, which is placed on the inner surface of the lateral lobe (or the intercalated platelet), is homologous with the uropod.
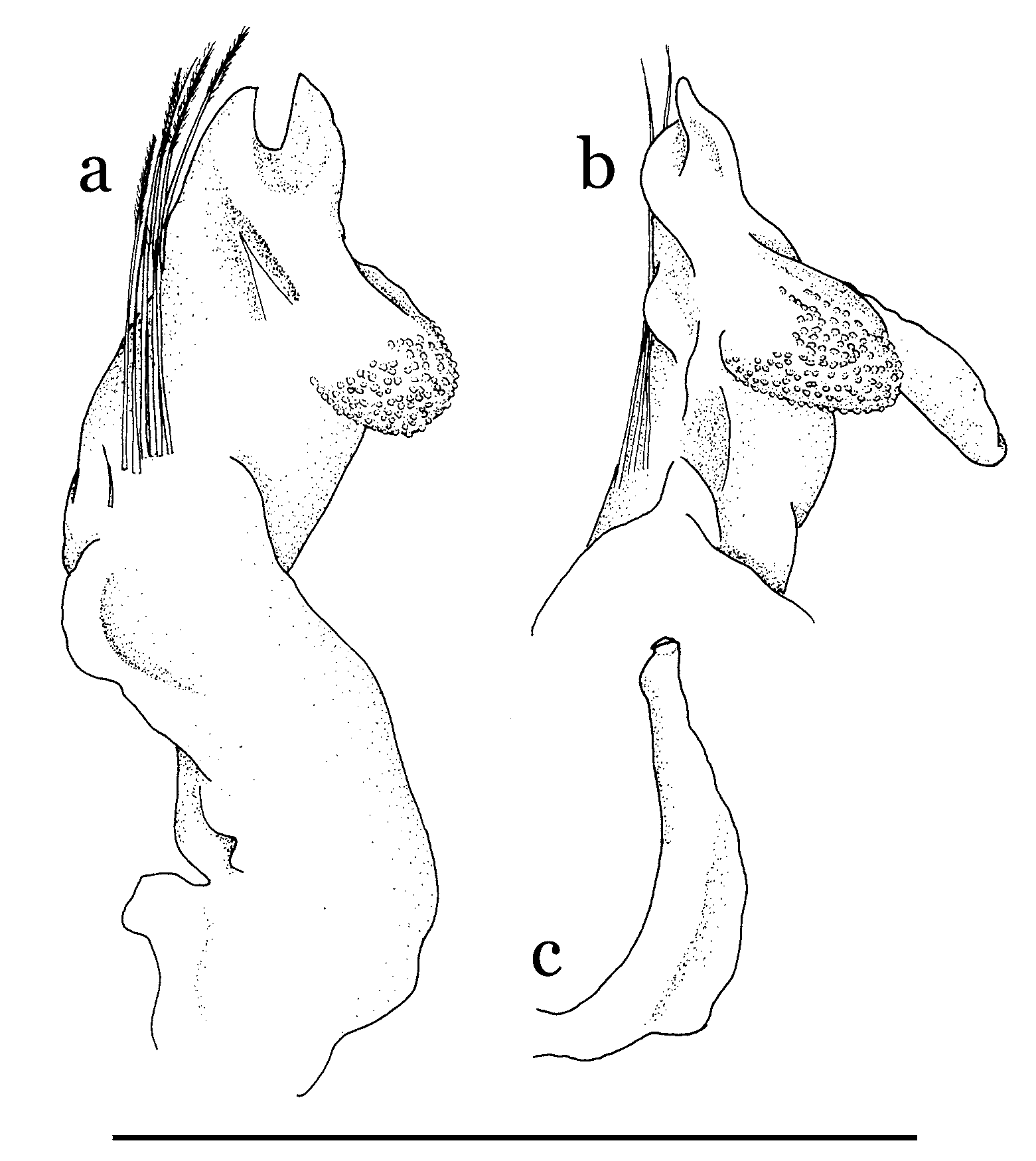
Our examination of the type material of H. carteri and H. robertsi new species, shows that the inner surface of the lateral lobe of the male pleotelson is domed and that it is externally surrounded by a thickened rim, forming a semicircular cavity ( Fig. 1 View FIGURE 1 b). When the abdomen is closed, the cavity overlays the sternal button ( Fig. 1 View FIGURE 1 a). This does not support the premise that the lateral lobe of the pleotelson is to accommodate the unusual tip of the G1 ( Figs. 2 View FIGURE 2 a, b, 5c, d). In fact, even when the abdomen is open, the G1 can easily be accommodated within the sterno-abdominal cavity ( Fig. 1 View FIGURE 1 a). The sternal buttons and the lateral cavities of the pleotelson function instead to “lock” the abdomen with the thorax. It should be noted that the G1 of H. carteri closely resembles that of Cancrocaeca xenomorpha (see also Guinot & Richer de Forges, 1997), and in the latter species, the male abdomen is more typical in shape, lacking the trilobite pleotelson, as well as sternal buttons, seen in Hymenicoides (Naruse, T., Ng, P.K.L. & Guinot, D., in manuscript).
Limnopilos naiyanetri Chuang & Ng, 1991 View in CoL ( type species of Limnopilos Chuang & Ng, 1991 View in CoL ), L. microrhynchus (Ng, 1995) View in CoL new combination, and L. sumatranus View in CoL new species, all possess the sternal button and the pleotelson lateral cavity ( Fig. 1 View FIGURE 1 c–f). The lateral cavities of these three species, however, are relatively smaller and the rim is more distal in position on the cavity when compared with those of H. carteri View in CoL and H. robertsi View in CoL . This may be due to the different shape of the G1 of Hymenicoides View in CoL . The lateral cavity of Hymenicoides View in CoL needs to be thickened along the external margins to effectively engage the sternal button, three sides (anterior, inner and posterior) which are surrounded by the G 1 in situ ( Fig. 1 View FIGURE 1 a). However, the sternal buttons of Limnopilos View in CoL are placed more distantly from the distal outer angle of the G1 ( Fig. 1 View FIGURE 1 c, e), and allow the lateral cavity to more easily lock onto the sternal button by way of the distally thickened rim.
The G1s of the Hymenicoides View in CoL species ( Fig. 2 View FIGURE 2 a, b, 5c, d) differ from those of Limnopilos View in CoL in several key aspects: they have a proportionately stouter shaft (vs. moderately stout in Limnopilos View in CoL ), more prominent distal inner processes (vs. moderately stout in Limnopilos View in CoL ) and with the distal outer angle more swollen than in Limnopilos View in CoL species ( Fig. 8 View FIGURE 8 c; Ng & Chuang, 1996: Fig. 21H; Ng, 1995: Fig. 14A, B).
The marked differences in the chela, abdomen and G1 lead us to now conclude that Limnopilos should be resurrected as a valid genus.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
Family |
Hymenicoides Kemp, 1917
| Naruse, Tohru & Ng, Peter K. L. 2007 |
L. microrhynchus
| Ng 1995 |
Limnopilos naiyanetri
| Chuang & Ng 1991 |
Limnopilos
| Chuang & Ng 1991 |