Drymarchon couperi ( Holbrook, 1842 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4138.3.7 |
|
publication LSID |
lsid:zoobank.org:pub:C7391621-50DB-4070-9BCF-3D00B49F291C |
|
DOI |
https://doi.org/10.5281/zenodo.5632172 |
|
persistent identifier |
https://treatment.plazi.org/id/B96C87BE-2305-5C26-FF6B-F9FCB4B40595 |
|
treatment provided by |
Plazi |
|
scientific name |
Drymarchon couperi ( Holbrook, 1842 ) |
| status |
|
Drymarchon couperi ( Holbrook, 1842)
Eastern Indigo Snake
( Figures 8–9 View FIGURE 8 View FIGURE 9 )
Coluber couperi Holbrook, 1842
Georgia couperi Baird & Girard 1853
Spilotes couperi (part) Cope 1860
Spilotes corais couperii (part) Lönnberg 1894 Compsosoma corais couperii (part) Cope 1900 Spilotes corais couperi (part) Brown 1901 Drymarchon corais couperi (part) Amaral 1929 Drymarchon couperi (part) Conant & Collins 1991 Holotype. Academy of Natural Sciences of Drexel University (ANSP 3937); although there is no known collector, locality, nor date assigned to this specimen, it is presumably collected by J.H. Couper ( McCranie 1980), from the Altamaha River ( Baird & Girard 1853), Wayne County, Georgia, USA ( Schmidt 1953).
Etymology. Drymarchon is derived from the Greek Drymos (meaning Oak Coppice or Forest) and archos (meaning Commander or Chief), referring to it being the chief of the oak woodland. The species epithet couperi is derived from the surname of J.H. Couper, who supposedly collected the holotype specimen.
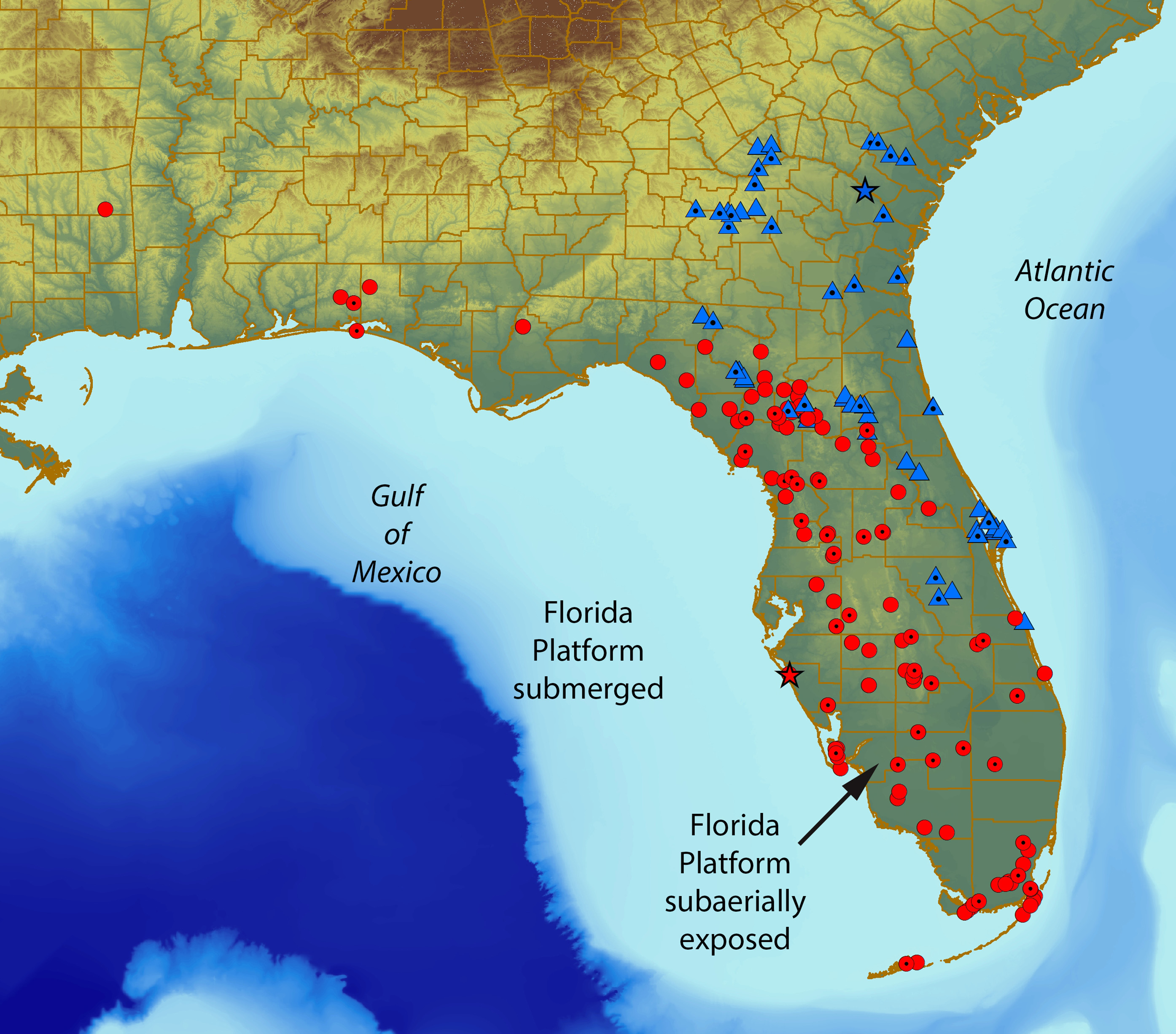
Distribution. Occurs from southeastern Georgia, southward into Florida from Suwannee and Alachua counties, southeastward to Marion, Osceola, and Indian River counties ( Fig. 7 View FIGURE 7 ).
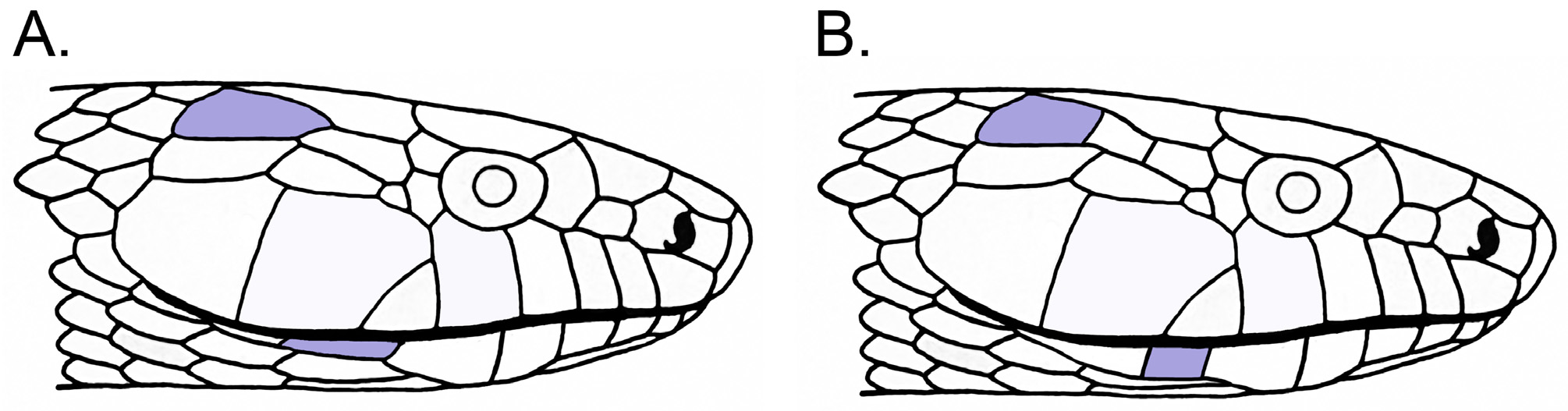
Diagnosis. Drymarchon couperi is distinguished by a suite of molecular and morphological features, including relatively longer and deeper head dimensions, longer and shallower 7th infralabials, and longer temporal scales. Overall, the presence of a longer and shallow 7th infralabial scale provides the best univariate predictor for this species ( Table 3 View TABLE 3 ; Fig. 5 View FIGURE 5 ). Based on both DNA ( Krysko et al. 2016) and morphology (specimens examined in this study) this species includes populations from southeastern Georgia southward along the Atlantic coast to central peninsular Florida.
Description of holotype. Adult male; Total Length 163.1 cm; Snout-vent Length 133.1 cm; Tail Length 30.0 cm; Head Length 51.3 mm; Head Width 25.2 mm. The relative head length (geometric shape variable; see Tables 2 and 3 View TABLE 3 ) is short (3.80) and relative head height is narrow (1.98). Supralabials 8/8 (left/right); Infralabials 9/9; Oculars 1+2/1+2; Temporals 2+2/2+2 (divided). The 7th infralabial is relatively long (0.65) and narrow (0.26) (geometric shape variable; see Tables 2 and 3 View TABLE 3 ) and the temporal scale is relatively short (0.78). The 5th and 7th supralabials are in contact with each other. Two pairs of chin shields, both of which are in contact with each other; posterior pair slightly narrower than anterior pair. The rostral visible from above, broader than high. Dorsal scales smooth and in rows at Mid-Body 17, Anterior 17 and Posterior 15; Ventral Scales 184; cloaca undivided; Subcaudal Scale Total 59 (all divided).
The specimen has faded from its likely original solid black dorsum. There is light pigment (likely reddish in real life) on the rostral, loreals, labials and chin shields that extend posteriorly onto the first three to four ventrals. The first 40 ventral scales are mottled or bicolored with different degrees of black (posteriorly) and light colored (distally) pigmentation with the remaining ventrals predominantly black. Subcaudals are entirely black.
Intraspecific variation. Ventrals range from 182–197 (mean = 188, n = 30); subcaudals range from 60–67 (mean = 63, n = 19); supralabials are arranged 8/8 (n = 28) and 7/7 (n = 2); infralabials are arranged 9/9 (n = 30); temporals are arranged 2 + 2 (n = 30), some individuals with smaller divided scales; oculars are arranged 1 + 2 (n = 30); and DSR are 15-17-15 (n = 17), 17-17-15 (n = 12), and 17-19-15 (n = 1).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Drymarchon couperi ( Holbrook, 1842 )
| Krysko, Kenneth L., Granatosky, Michael C., Nuñez, Leroy P. & Smith, Daniel J. 2016 |
couperi
| Baird & Girard 1853 |
Coluber couperi
| Holbrook 1842 |