Sylvicanthon securus ( Schmidt, 1920 ), 2018
|
publication ID |
https://doi.org/10.5852/ejt.2018.467 |
|
publication LSID |
lsid:zoobank.org:pub:8D27AAB8-B7F2-424C-B1A6-66FEFA66EDFF |
|
DOI |
https://doi.org/10.5281/zenodo.3846305 |
|
persistent identifier |
https://treatment.plazi.org/id/A72C87FB-FF1F-FF2F-0EEA-08E60A7B956F |
|
treatment provided by |
Valdenar |
|
scientific name |
Sylvicanthon securus ( Schmidt, 1920 ) |
| status |
comb. nov. |
Sylvicanthon securus ( Schmidt, 1920) View in CoL comb. nov.
Figs 6F View Fig , 11C View Fig , 15O View Fig , 19A View Fig , 20 View Fig , 39 View Fig , 40A View Fig , 41 View Fig
Canthon securus Schmidt, 1920: 131 View in CoL , 133.
Canthon securus View in CoL – Schmidt 1922: 64, 80. — Balthasar 1939: 188. — Halffter & Martínez 1977: 63. — Krajcik 2012: 64.
Canthon securum – Blackwelder 1944: 201.
Glaphyrocanthon ( Glaphyrocanthon) securus – Pereira & Martínez 1956: 126, 128. — Martínez et al. 1964: 5–6, 9, 14, 20. — Vulcano & Pereira 1964: 664.
Sylvicanthon cf. securus – Larsen 2012: “92”, 99.
Etymology
Possibly from the Latin ‘ securis ’, meaning ‘axe’ or ‘hatchet’, in reference to the shape of protibiae, which have a strong internal expansion. The Latin word ‘ securus ’, ‘free of doubts’, does not seem to be the origin of this name, unless Schmidt (1920) has referred to the fact that this species is extremely different from the others and, therefore, he had no doubts about its validity.
Material examined
Lectotype (here designated)
SURINAME: ♂, (“Surinam”, “S ecurus ”, “S ecurus / A. Schm. ”, “ Glaphyrocanthon / securus / (Schm.) / P. Pereira det. 60 ”, “3205 / E92 +”, “S ecurus / Schmidt”, “34 / 56”, “NHRS-JLKB / 000021093” “LECTOTYPE ♂ / Canthon / securus / A. Schmidt / des. F. Z. Vaz-de-Mello, 20 14 ”) ( NHRS) ( Fig. 11 H View Fig ).
Additional material (22 ♂♂, 27 ♀♀, 1 unsexed specimen)
BRAZIL: 1 ♂, no further data ( BMNH). – Amazonas: 2 ♀♀, Manaus, ZN 03, Km 41, 1996–1997 [ Alouatta seniculus (?) dung], Ellen Andressen leg. ( CEMT); 1 ♂, same collecting data as for preceding ( INPA). – Pará: 1 ♂, Almeirim, Monte Dourado, 01º01’ S, 52º44’ W, 150 m, Jul. 2004, dung pitfall, T.A. Gardner leg. ( CEMT).
FRENCH GUIANA: 4 ♂♂, 1 ♀, SEAG leg. ( CEMT); 1 ♂, Cayenne, Kourou, Rte. Cayenne-Sinnamary, RN1, PK84, Jan. 2013, flight interception trap, SEAG leg. ( CEMT); 1 ♀, Cayenne, Montsinéry-Tonnegrande, Montagne des Chevaux, 04º44’56″ N, 52º26’28″ W, 75 m, 30 Oct. 2011, SEAG leg. ( CEMT); 1 ♀, Cayenne, Montsinéry-Tonnegrande, Montagne des Chevaux, 04º44’56″ N, 52º26’28″ W, 75 m, 27 Jan. 2013, SEAG leg. ( CEMT); 1 ♂, Cayenne, Montsinéry-Tonnegrande, Montagne des Chevaux, 04º44’56″ N, 52º26’28″ W, 75 m, 21 Dec. 2013, SEAG leg. ( CEMT); 2 ♂♂, Cayenne, Régina , [Réserve naturelle nationale des] Nouragues, Nov. 1996, F. Feer leg. ( CEMT); 1 unsexed specimen, Cayenne, Régina , [Réserve naturelle nationale des] Nouragues, Oct. 2001, Kelly leg. ( BMNH); 2 ♂♂, 3 ♀♀, Cayenne, Régina , [Réserve naturelle nationale des] Nouragues, Inselberg, 04º05′ N, 52º41′ W, 411 m, 4 Apr. 2010, SEAG leg. ( CEMT); 1 ♀, Cayenne, Régina , [Réserve naturelle nationale des] Nouragues, Inselberg, 04º05′ N, 52º41′ W, 411 m, 13 Oct. 2012, SEAG leg. ( CEMT); 1 ♂, Cayenne, Régina , [Réserve naturelle nationale des] Nouragues, Inselberg, 04º05′ N, 52º41′ W, 411 m, 14 Jun. 2013, SEAG leg. ( CEMT); 1 ♂ (dissected), Cayenne, Régina , [Réserve naturelle nationale des] Nouragues, Inselberg, 04º05′ N, 52º41′ W, 411 m, 19 Sep. 2013, SEAG leg. ( CEMT); 2 ♀♀, Cayenne, Roura, Montagne de Kaw, Nov. 1996, F. Feer leg. ( CEMT); 1 ♀, Cayenne, Roura, Montagne de Kaw, 3 Feb. 2007, F. Feer leg. ( CEMT); 1 ♀, Cayenne, Roura, Montagne de Kaw, 17 Feb. 2009, F. Feer leg. ( CEMT); 1 ♀, Cayenne, Roura, Montagne de Kaw, 18 Feb. 2009, F. Feer leg. ( CEMT); 2 ♀♀, Saint-Laurent- du-Maroni, Maripasoula, Saül, Bélvédère de Saül, 03º37′22″ N, 53º12′57″ W, 326 m, 11 Jan. 2011, SEAG leg. ( CEMT); 1 ♂, 2 ♀♀, Saint-Laurent-du-Maroni, Maripasoula, Saül, Bélvédère de Saül, 03º37′22″ N, 53º12′57″ W, 326 m, 27 May 2011, SEAG leg. ( CEMT); 1 ♀, Saint-Laurent-du-Maroni, Maripasoula, Saül, Bélvédère de Saül, 03º37′22″ N, 53º12′57″ W, 326 m, 15 Jun. 2011, SEAG leg. ( CEMT); 1 ♀, Saint-Laurent-du-Maroni, Maripasoula, Saül, Bélvédère de Saül, 03º37′22″ N, 53º12′57″ W, 326 m, 23 Jun. 2011, SEAG leg. ( CEMT); 1 ♂, 1 ♀, Saint-Laurent-du-Maroni, Maripasoula, Saül, Bélvédère de Saül, 03º37′22″ N, 53º12′57″ W, 326 m, 28 Jul. 2011, SEAG leg. ( CEMT); 3 ♀♀, Saint-Laurent-du-Maroni, Maripasoula, Saül, Bélvédère de Saül, 03º37′22″ N, 53º12′57″ W, 326 m, 9 Sep. 2011, SEAG leg. ( CEMT); 1 ♂, 1 ♀, Saint-Laurent-du-Maroni, Maripasoula, Saül, Bélvédère de Saül, 15 Sep. 2011, SEAG leg. ( CEMT); 2 ♂♂, 2 ♀♀, Saint-Laurent-du-Maroni, Maripasoula, Saül, Bélvédère de Saül, Grand Boeuf Mort, 10 Oct. 2007, SEAG leg. ( CEMT).
No data: 1 ♂ ( ISNB – “Coll. J. Thomson”); 1 ♂ ( MNHN).
Description
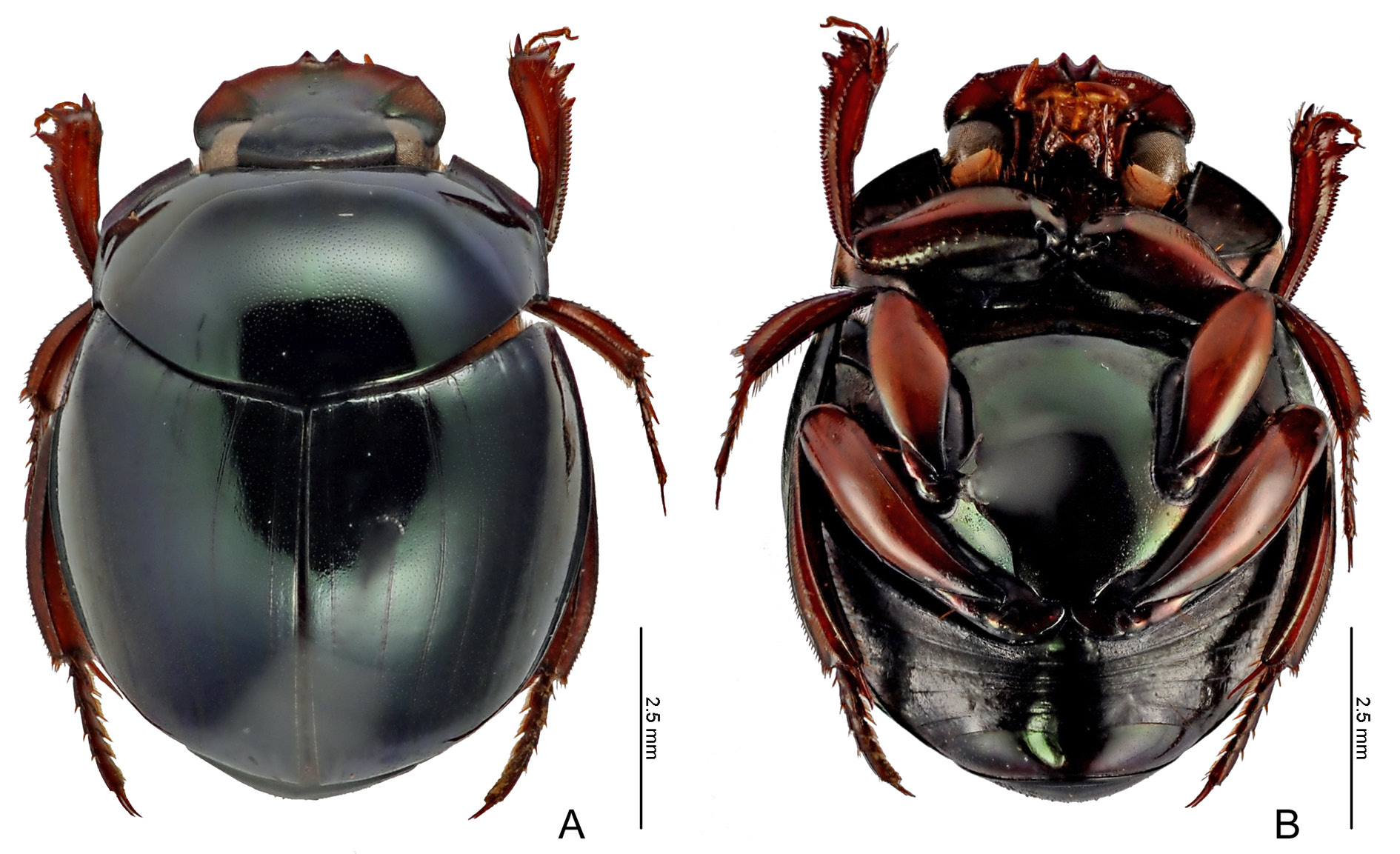
COLOURATION. Head bicolour, with wide purplish or coppery area covering in general outer edge (apex of clypeus and genae) and posterior region (frons, posterior portion of clypeus, and, occasionally, part of genae) bright green. Pronotum entirely green, never with purplish or coppery spot. Elytra green, with striae contrasting or not. Metaventrite with greenish shine at centre and purplish or coppery at rest of tegument. Meso- and metafemora yellowish or orangish; profemur slightly darker than others. Pygidium bright green.
HEAD. Tegument with strong alveolar microsculpture throughout dorsal surface; micropunctation strongly marked on frons and progressively more effaced towards outer edge of head ( Fig. 6F View Fig ). Clypeus triangular, with very acute pair of apical teeth; each tooth individually margined at base. Genae with clear denticle immediately behind clypeal-genal juncture (denticle sometimes reduced by wear). Posterior
edge of head usually with margin at centre (margin absent adjacent to eyes); in some specimens, margin very fine and almost imperceptible, or even absent.
THORAX. Pronotum with shiny tegument and dense, well-marked micropunctation at centre; towards sides, micropunctation progressively less dense and well marked, and sometimes absent; tegument among micropunctation smooth, without microsculpture; alveolar microsculpture restricted to very narrow strip of tegument on anterior edge of pronotum and anterolateral angles. Posterior edge with very fine transverse line at centre (usually extending little beyond the second elytral stria). Hypomeral cavity covered by long yellowish setae; external margin simple, without any trace of tubercle (occasionally, with only very weak callosity marking location of tubercle). Metaventrite glabrous at centre and with a group of very few yellowish setae on sides near external extremity of metacoxae; anterior region of metaventrite with tegument with strong rivose microsculpture; centre and posterior region with strong and dense micropunctation among smooth tegument, without any trace of microsculpture.
LEGS. Profemora with tegument with diffuse rivose microsculpture, but hardly visible. Protibiae very narrow at basal half and with strong angulose expansion at apical half of internal edge, making apical half twice as wide as basal half; at apical fourth), with three small acute teeth on external edge – the most basal tooth distinctly smaller than others ( Fig. 11C View Fig ). Mesofemora margined anteriorly only at basal half; unmargined portion of anterior edge with row of very short setae; posterior margin absent; tegument smooth and lustrous, except for narrow strip on anterior edge with effaced rivose microsculpture. Metafemora margined only anteriorly, posterior margin absent; apical third of anterior edge covered by row of setae; tegument almost entirely smooth and lustrous, with weak traces of rivose microsculpture only at some regions; without coarse elongate punctation at base. Metatarsomeres II and V subequal in length and longer than the others; metatarsomere IV shorter than the others.
ELYTRA. With seven very narrow visible striae: first five striae well marked, finely carinulate, and distinctly widened at base; sixth and seventh striae progressively more effaced and interrupted; seventh stria occasionally vestigial or even completely absent; all striae lack carinulae before reaching apex of elytra. Interstriae with dense, well-marked micropunctation at centre among smooth tegument; without any trace of microsculpture.
ABDOMEN. Tegument of ventrites I–V with strong rivose microsculpture; ventrite VI bright, without microsculpture, and with sparse micropunctation at centre, and with very weak rivose microsculpture on sides. Both sexes without lateral foveae. Pygidium with dense, well-marked micropunctation among tegument mostly smooth, with rivose microsculpture restricted to narrow strip adjacent to basal margin.
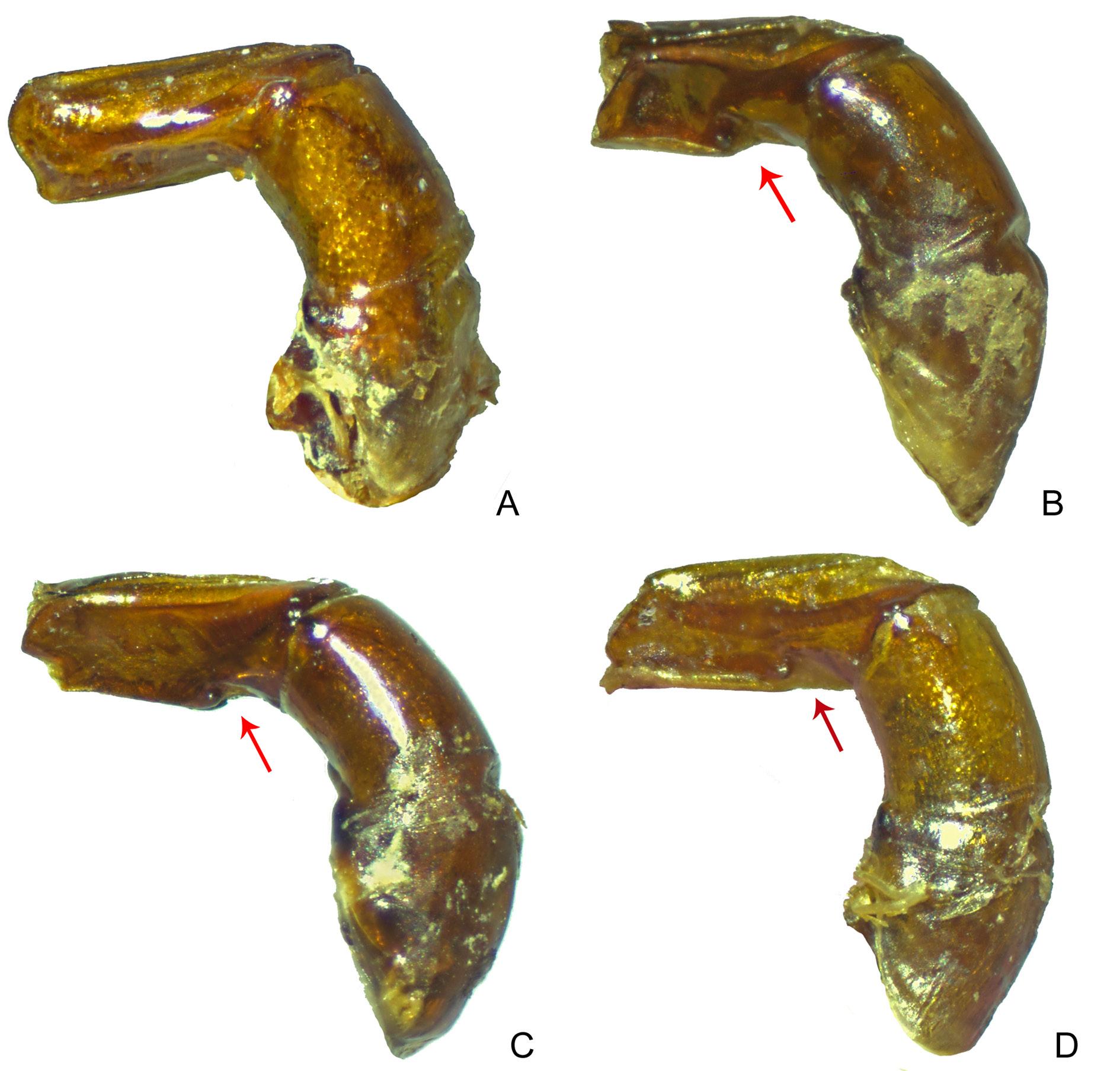
AEDEAGUS. Parameres simple, very long (only slightly shorter than phallobase), without ventral keel or notch, and with no noticeable asymmetry: both parameres with flat external face ( Fig. 19A View Fig ).
SEXUAL DIMORPHISM. Males: Protibial spur broad and bifid, with internal projection broad and much longer than external projection, which is short and spiniform ( Fig. 15O View Fig ). Ventrite VI strongly narrowed at centre by emargination on posterior edge; ventrite V with weak medial flange on posterior edge covering anterior edge of ventrite VI. Females: Protibial spur spiniform. Ventrite VI very broad at centre, without emargination on posterior edge; ventrite V as in males.
Measurements
Males (N = 11). TL: AV: 6.1 ± 0.14; MX: 6.9; MN: 6.5. EW: AV: 5.2 ± 0.28; MX: 5.9; MN: 4.8. PrL: AV: 2.3 ± 0.10; MX: 2.4; MN: 2.1. PrW: AV: 4.5 ± 0.22; MX: 4.9; MN: 4.1. PgL: AV: 1.0 ± 0.07; MX: 1.2; MN: 0.9. PgW: AV: 2.1 ± 0.10; MX: 2.3; MN: 2.
Females (N = 11). TL: AV: 6.8 ± 0.21; MX: 7.0; MN: 6.3. EW: AV: 5.0 ± 0.10; MX: 5.2; MN: 4.9. PrL: AV: 2.1 ± 0.07; MX: 2.3; MN: 2.1. PrW: AV: 4.2 ± 0.10; MX: 4.4; MN: 4.1. PgL: AV: 1.0 ± 0.08; MX: 1.1; MN: 0.9. PgW: AV: 2.1 ± 0.11; MX: 2.3; MN: 1.9.
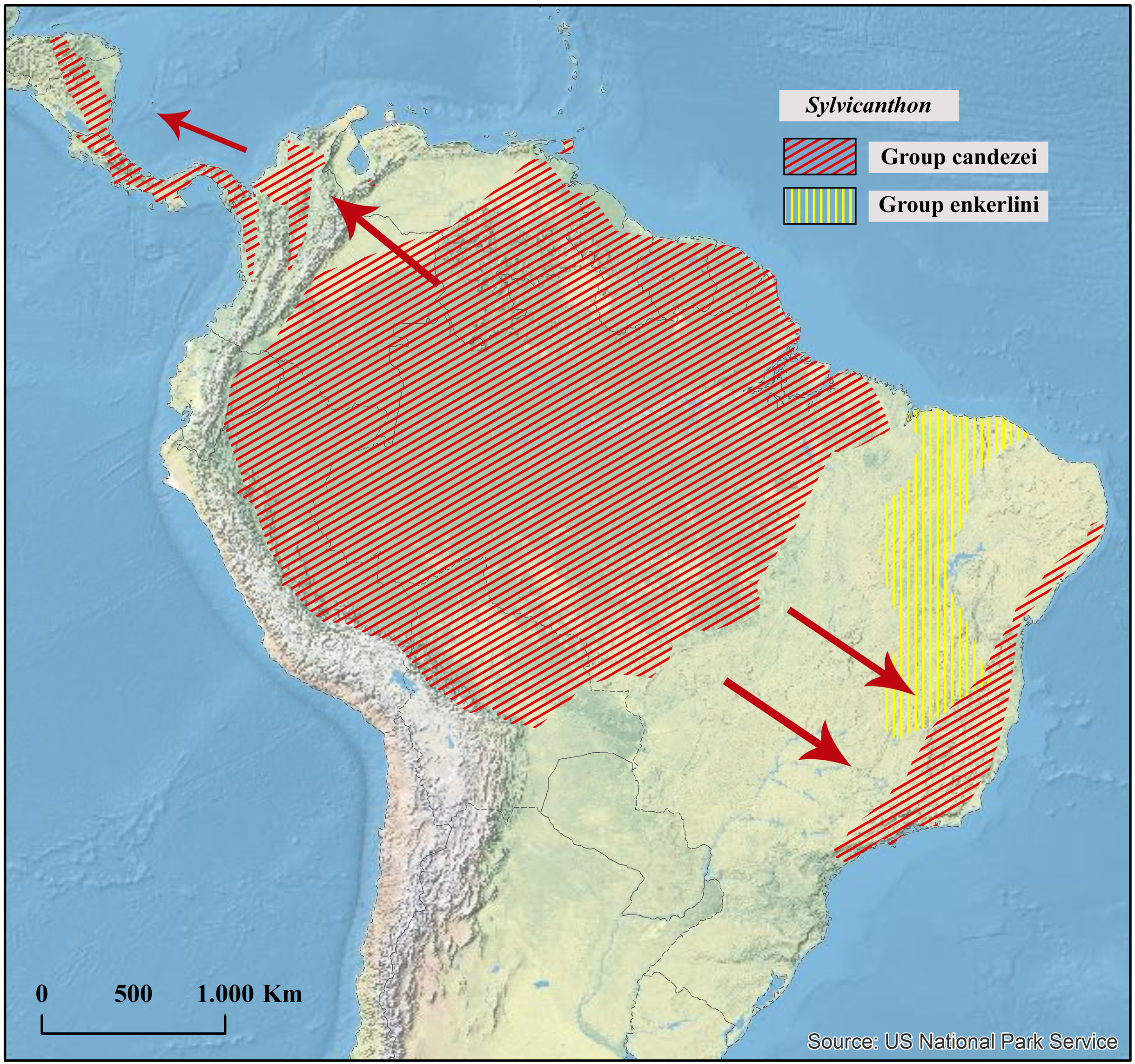
Geographical distribution
Northern Amazonia in Suriname, French Guiana and Brazil.
Ecoregions
Guianan Moist Forests, Marajó Varzea, Uatuma-Trombetas Moist Forests, Japurá-Solimões-Negro Moist Forests
Collecting sites ( Fig. 41 View Fig )
SURINAME. Sipaliwini: Coeroeni.
FRENCH GUIANA. Cayenne (Kourou; Montsinéry-Tonnegrande: Montagne des Chevaux Régina: Réserve naturelle nationale des Nouragues; Roura: Montagne de Kaw), Saint-Laurent-du-Maroni (Maripasoula: Saül).
BRAZIL. Amazonas: Manaus. Pará: Almeirim (Monte Dourado).
Intraspecific variation and taxonomic discussion
It is interesting to note that S. securus is one of the rarest and, at the same time, one of the most easily recognizable species of Sylvicanthon , being the most isolated species in the genus in terms of morphology. Despite that, most of the studied specimens of S. securus were either unidentified in collections or mingled among specimens of S. seag sp. nov. In fact, throughout its distribution range – i.e., in the Guiana Shield and the Amazon region north of the Amazon River – S. securus is sympatric with S. seag sp. nov., a species that in turn extends its distribution far beyond this area both northwestwards, reaching Venezuela and Trinidad, and eastwards, reaching the Amazon fragments of the Brazilian state of Maranhão ( Fig. 41 View Fig ). Besides, at first glance, S. securus and S. seag sp. nov. may be confused by colouration which, in the Guianas, is almost always shiny green in both species ( Figs 37C View Fig , 39A View Fig ) and by the number of protibial teeth, three ( Fig. 11C View Fig ). Nonetheless, at a closer look, the differences between S. securus and S. seag sp. nov. become evident.
The easiest way to differentiate both species is by examining the protibiae: S. seag sp. nov. has their internal margin straight and simple ( Fig. 11 View Fig H–I), while S. securus has them strongly expanded, with the apical half of the protibiae almost twice as wide as the basal half ( Fig. 11C View Fig ). In fact, the expanded internal margin of the protibiae is a very common feature in Sylvicanthon , being present in all the four species of the furvus subgroup ( S. obscures ( Fig. 11D View Fig ), S. mayri sp. nov., S. monnei sp. nov., and S. furvus ( Fig. 11E View Fig )), in S. bridarollii ( Fig. 11 View Fig F–G; the other species of the bridarollii subgroup have a simple internal margin), and S. enkerlini ( Fig. 11A View Fig ), but it is much more pronounced in S. securus than in any other species of the genus (something already noted by Schmidt (1922) and Balthasar (1939)). Other groups of Deltochilini also show this feature, e.g., several species of Glaphyrocanthon and Francmonrosia .
The overall texture of the tegument is distinct between S. seag sp. nov. and S. securus : in the former species, the pronotum, metaventrite, elytra and pygidium are covered by a strong alveolar microsculpture which obliterates the micropunctation (which, consequently, is very weak). In S. securus , on the other hand, there is no trace of microsculpture on the pronotum, at the centre of metaventrite, on the elytra or at the centre of the pygidium (in these latter two cases, sometimes there is a very diffuse indication of microsculpture), and the micropunctation is strong and clearly visible. Sylvicanthon securus also differentiates from S. seag sp. nov. by the presence of a fine margin at the centre of the posterior edge of the head in the majority of the specimens, while all the examined individuals of the latter species have the posterior edge of the head without any trace of margination. Nonetheless, this characteristic is largely variable in S. securus : the fine margin may be clearly present and almost reaches the eyes, or it may be gradually shorter or even almost absent.
Other morphological structures distinguishing S. securus from S. seag sp. nov. are the shape of the edge of the clypeus, which is completely rounded in S. seag sp. nov. ( Fig. 6E View Fig ) and is slightly sinuous adjacently to the apical teeth in S. securus ( Fig. 6F View Fig ); the basal margin of the clypeal teeth, which is divided into two non-continuous parts (i.e., each tooth has its own basal margin) in S. securus , and is one-piece (i.e., a single margin covers the base of both teeth) in S. seag sp. nov.; and the shape of the male protibial spur, which has the internal branch much longer than the external one in S. securus ( Fig. 15N View Fig ), whereas the opposite condition is seen in S. seag sp. nov. ( Fig. 15J View Fig ). Clear differences also exist in the shape of the parameres: in S. securu comb. nov., they are simple, fine and laterally flat, without any ventral keel or notch ( Fig. 19A View Fig ). In turn, in S. seag sp. nov. the parameres are strongly asymmetrical (the external face of the right paramere flat and the external face of the left paramere excavated) and, in lateral view, there is a ventral keel strongly projected, giving a squarish appearance to the apical half of the parameres and it also has a notch posteriorly to that keel ( Fig. 18B View Fig ).
Differences are seen in the colouration of fully mature specimens (i.e., excluding teneral individuals): in the Guianas, S. seag sp. nov. has an overall dorsal colouration shiny green similar to that seen in S. securus , but it differs from this latter species in having the head and, occasionally, the pronotum covered by a purplish spot ( Fig. 37C View Fig ), while in S. securus the pronotum is always as green as the elytra, and head possesses a narrow purplish spot limited to the the apex of the clypeus ( Fig. 39A View Fig ). Ventrally, the differences are seen on the colouration of the metaventrite – which, although sometimes mostly coppercoloured, always shows some greenish reflections at the centre in S. securus ( Fig. 39B View Fig ), while it is always coppery without any greenish reflection in S. seag sp. nov. ( Fig. 37D View Fig ) – and of the profemora – which are light brown in S. securus , and much darker brown in S. seag sp. nov. Farther south, in Brazil, individuals of S. seag sp. nov. have a very distinct colouration, with bluish elytra and a purplish pronotum ( Fig. 37A View Fig ), in which they do not resemble in anything the bright green colouration of S. securus .
Comments
Schmidt (1920) did not cite the number of specimens he examined for the description of S. securus , but from his text, it is possible to conclude that he had only males at his disposal, since he described the male shape of the protibial spur, but did not cite how this structure was in females. The only specimen from the type series of S. securus found by us was a male deposited in the NHRS which is here designated as lectotype.
One of the specimens found at the BMNH bears a circular blue label handwritten “ 54 / 60 ”, without any further indication. According to Max Barclay (personal communication to MC, 2015), this code refers to a large acquisition of several Brazilian dung beetle species made by the museum from the French entomologist Henri Jekel ( 1816–1891). This specimen also has another rectangular white label handwritten “ N… / serricornis / n… ”, probably an apocryphal nomen in litteris that we are unware of having been applied to any other specimen or cited in any publication.
Natural history
Sylvicanthon securus is a rare species, inhabiting lowland forests in the northern Amazon region (with records from 75 up to 411 m of altitude), where it is sympatric with S. seag sp. nov. Most of the specimens of S. securus studied for this work originated from French Guiana and, of the two species of Sylvicanthon that occur there, S. securus was collected in an evident lower abundance: a total of 43 specimens of S. securus were caught in French Guiana, in comparison to 601 S. seag sp. nov., a number almost 15 times higher. Taking the series of specimens studied for this work into account, it was possible to see that, in individual collecting episodes, the ratio between the abundance of S. seag sp. nov. and S. securus varied from 3:1 up to 65:1. From Suriname, in turn, only the lectotype of S. securus is known, while 25 specimens of S. seag sp. nov. were caught in that country.
With such a discrepancy in the relative abundance between these two species, it is really remarkable that S. securus was described still in the early 20 th century, while S. seag sp. nov. had to wait until now to have its condition as a distinct species recognized. However, an obvious question arises from these observations: what is the ecological factor, or conjunct of factors, responsible for the remarkable difference in abundance between S. seag sp. nov. and S. securus? Nonetheless , it would be fair to question whether this apparent rarity of S. securus is not a simple artefact generated by some unknown idiosyncratic life habit of the latter species; for example, some other food preference than primate dung (i.e., human faeces used to bait pitfalls). Be that as it may, only with more research on the biology of both S. securus and S. seag sp. nov. it will be possible to give a proper answer to these questions.
Judging from the specimen labels, we know that S. securus was collected in pitfall traps baited with human faeces and flight interception traps. Besides, the two females from Manaus (Amazonas, Brazil) were attracted to howler monkey dung; although their labels indicate that the primate was an Alouatta seniculus (Linnaeus, 1766) , the correct identity of this species should be A. macconnelli Elliot, 1910 , or, less probable, A. nigerrima Lönnberg, 1941 , the only two species of the A. seniculus complex present in that area ( Gregorin 2006). Regarding the species’ annual phenology, adults of S. securus were collected throughout the year, including the months of January, February, May, June, July, September, and October.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
SubPhylum |
Hexapoda |
|
Class |
|
|
Order |
|
|
SubOrder |
Polyphaga |
|
SuperFamily |
Scarabaeoidea |
|
Family |
|
|
SubFamily |
Scarabaeinae |
|
Tribe |
Deltochilini |
|
Genus |
Sylvicanthon securus ( Schmidt, 1920 )
| Cupello, Mario & Vaz-De, Fernando Z. 2018 |
Canthon securus
| Halffter G. & Martinez A. 1977: 63 |
| Balthasar V. 1939: 188 |
| Schmidt A. 1922: 64 |
Canthon securus
| Schmidt A. 1920: 131 |