Hypericum hengshanense
|
publication ID |
https://doi.org/10.1016/j.phytochem.2022.113500 |
|
persistent identifier |
https://treatment.plazi.org/id/9F258790-3D3A-0567-FFA6-FDC20094FA95 |
|
treatment provided by |
Felipe |
|
scientific name |
Hypericum hengshanense |
| status |
|
2.1. Isolation and structural elucidation of MAPs from H. hengshanense View in CoL
The dried whole plants of H. hengshanense ( 1.8 kg) were extracted exhaustively with MeOH. Extracts were subjected to silica gel column chromatography, followed by a UV-guided fractionation ( 290 nm). The separation and purification were carried out by MCI GEL CHP20P (a highly porous styrene-divinylbenzene polymer resin), octadecyl silica (ODS), Sephadex LH-20, and silica gel column chromatography, leading to the isolation of 25 MAPs ( Fig. 1).
Hyphengshanol A ( 1) was obtained as a pale yellowish powder. Its molecular formula was defined as C 21 H 28 O 4 based on a negative ion at m / z 343.1915 [M – H] ‒ (calcd for 343.1915) in the HR-ESI-MS spectrum, suggesting eight degrees of unsaturation. A characteristic UV absorption band of acylphloroglucinol was observed at 288 nm in the UV spectrum. In the 1 H NMR and 13 C NMR spectra, a group of resonances belonging to the acylphloroglucinol moiety were also found, [ δ H 5.79 (s, 1H, H-1), 3.73 (m, 1H, H-2 ′), 1.93 (m, 1H, H-3 ′ a), 1.83 (m, 1H, H-3 ′ b), 0.90 (t, 3H, J = 6.6 Hz, H-4 ′), 1.16 (d, 3H, J = 6.6 Hz, H-5 ′), 6.56 (s, 1H, H-2-OH), 12.95 (s, 1H, H-4-OH); δ C 96.5 (C-1), 159.4 (C-2), 104.5 (C-3), 157.8 (C-4), 104.3 (C-5), 164.3 (C-6), 210.1 (C-1 ′), 45.9 (C-2 ′), 27.1 (C- 3 ′), 12.0 (C-4 ′), 16.6 (C-5 ′)]. The aromatic proton in the acylphloroglucinol moiety presents a resonance at δ H 5.72 when it is located at C-1 or a signal around δ H 5.88–5.96 when it is located at C-3 ( Schmidt et al., 2012a). The chemical shift of the aromatic proton at δ H 5.79 (s, 1H, H-1) suggested that it was located at C- 1 in the acylphloroglucinol moiety. Besides the acylphloroglucinol moiety, HSQC and 13 C NMR data revealed that there were 10 carbons left, suggesting the presence of three methyls, two methylenes, three methines, a quaternary carbon, and an oxygenated tertiary carbon ( Table 1). These data suggested that compound 1 was a polyprenylated acylphloroglucinol. In addition, the acylphloroglucinol fragment accounts for five degrees of unsaturation, indicating that there are three rings in the polyprenyl moiety of 1.
In the COSY spectrum, homonuclear vicinal coupling correlations between H-7 and H-8, H-8 and H-13, H-13 and H-12, and H-12 and H-11 suggested the connections of the C-7‒C-8‒C-13‒C-12‒C-11 fragment. As shown in Fig. 2 View Fig , HMBC crosspeaks from H-7 to C-4, C-5, and C-6 indicated the polyprenyl fragment attached to the acylphloroglucinol at C-5. H-15 and H-16 both showed HMBC correlations to C-7 and C-13, indicating that the two methyls locate at C-14 and established the fourmember ring. HMBC crosspeaks from H-10 to C-8, C-9, and C-11 determined the five member ring. Another ring in the polyprenyl moiety was suggested to be an oxygen heterocycle fused to the phloroglucinol, according to the degrees of unsaturation, the oxygenated sp 3 carbon (C- 9, δ C 84.6), and the HMBC crosspeaks from H-7 to C-5 and C-6 and from H-10 to C-8 and C-9. Thus establishing the planar structure of 1 as shown in Fig. 2 View Fig . It bears a rare 6/6/5/4 core. Only three analogs hypersines A–C were previously reported to possess such a skeleton ( Liu et al., 2021). Interestingly, hypersines A–C were isolated from H. elodeoides , a botanical relative of H. henshanense , which was a species in the Hypericum sect. Elodeoida .
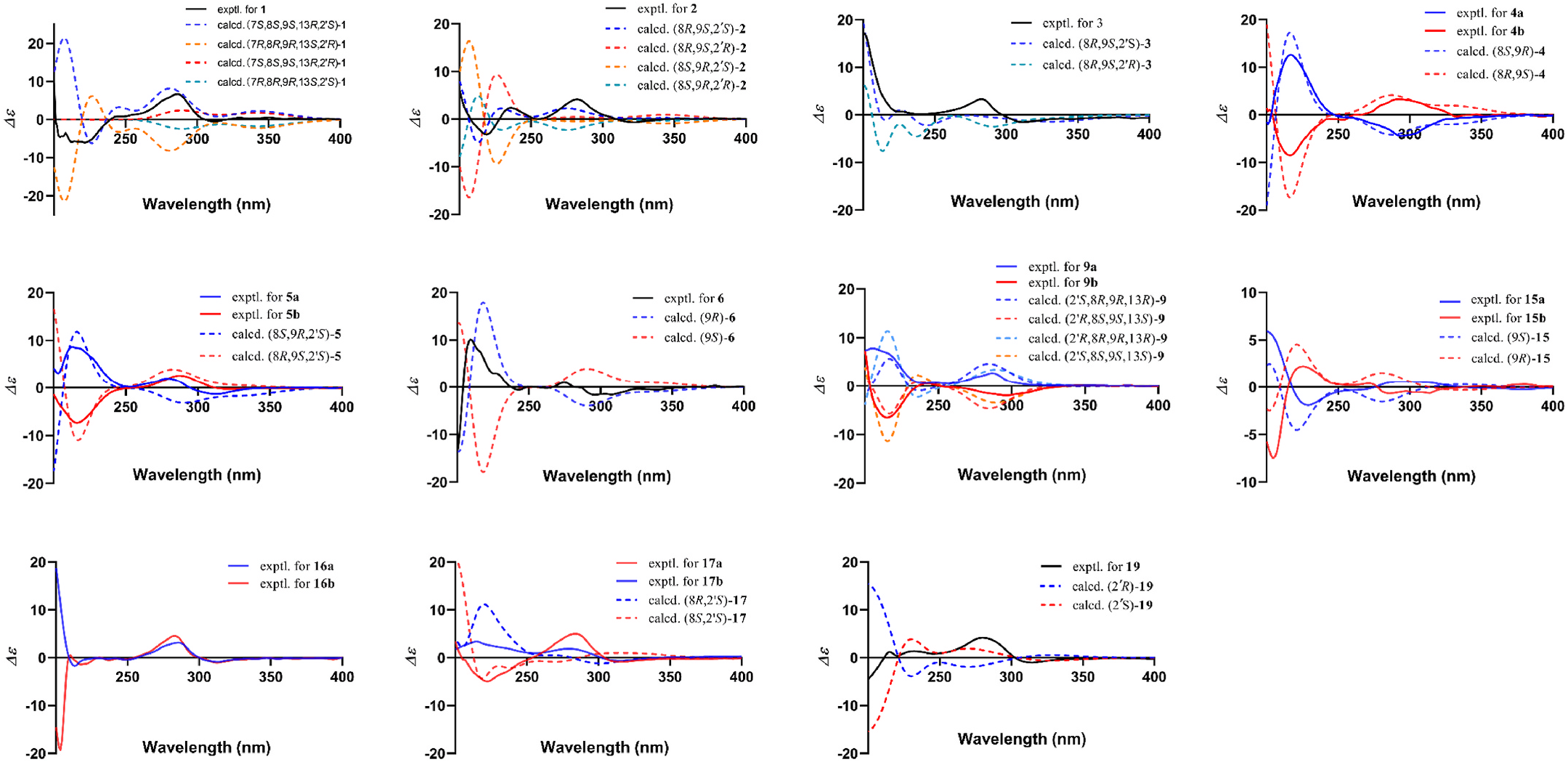
The NOESY correlations between H-10 and H-7, H-8, and H- 13 in 1 indicated that they were situated at the same plane, thus defining the orientation of these substituents as shown in Fig. 2 View Fig . However, the relative configuration of C-2 ′ could not be determined due to the absence of conclusive evidence. To elucidate the absolute configuration, ECD calculations of four potential configurations, (7 S,8 S,9 S,13 R,2 ′ S), (7 S,8 S,9 S,13 R,2 ′ R), (7 R,8 R,9 R,13 S,2 ′ R), and (7 R,8 R,9 R,13 S,2 ′ S)- 1 were carried out. Comparison of the calculated and experimental spectra of 1 were shown in Fig. 3 View Fig . The experimental ECD curve presented similar cotton effects to (7 S,8 S,9 S,13 R,2 ′ S). Both showed a negative cotton effect at 220 nm and a positive cotton effect at 286 nm, thus assigning the absolute configuration of 1.
Hyphengshanol B ( 2) was obtained as a pale yellowish powder. Its negative-ion m / z 361.2007 [M – H] ‒ (calcd for 361.2020) in the HR-ESI-MS spectrum corresponded to a molecular formula of C 21 H 30 O 5. The presence of the acylphloroglucinol fragment was confirmed by an absorption band 295 nm in the UV spectrum and a group of characteristic resonances in the 1 H NMR and 13 C NMR spectra, [ δ H 5.90 (s, 1H, H-3), 3.60 (m, 1H, H-2 ′), 1.45 (m, 1H, H-3 ′ a), 1.82 (m, 1H, H-3 ′ b), 0.92 (t, 3H, J = 7.2 Hz, H-4 ′), 1.15 (d, 3H, J = 7.2 Hz, H-5 ′); δC 101.9 (C-1), 166.0 (C- 2), 96.5 (C-3), 158.3 (C-4), 104.3 (C-5), 162.6 (C-6), 208.6 (C-1 ′), 45.2 (C-2 ′), 26.6 (C-3 ′), 11.9 (C-4 ′), 16.3 (C-5 ′)]. The deshielded chemical shift of the aromatic proton at δ H 5.90 (Δ + δ H 0.11) suggested that the proton was located at C-3. Combined with the HSQC and 13 C NMR data, there were 10 carbons left, classified as: three methyls [ δ H 1.33 (3H, s), δ C 23.0; δ H 1.64 (3H, s), δ C 17.8; δ H 1.70 (3H, s), δ C 25.9], three methylenes [ δ H 2.12 (1H, m), 2.19 (1H, m), δ C 22.1; δ H 3.04 (1H, m), 3.06 (1H, m), δ C 26.4; δ H 1.58 (2H, m), δ C 37.7], an oxygenated methyne [ δ H 4.80 (t, 1H, J = 9.0 Hz), δ C 91.1], a methine group [ δ H 5.14 (br s, 1H), δ C 123.9], an olefinic carbon at δ C 132.6, and an oxygenated tertiary carbon at δ C 73.7. These data suggested that compound 2 was a MAP which consists of two isoprenyls and an acylphloroglucinol moiety.
The COSY correlations of H-11/H-12 and H12/H13 combined with the HMBC crosspeaks from H-15 and H-16 to C-13 suggested that an isoprenyl group was attached to C-11. HMBC crosspeaks from H-7 to C- 5, C-6, and C-8 and from H-10 to C-8, C-9, and C-11 established the fragment from C-5 to C-11. A furan ring rather than a pyran ring, which fused to the phloroglucinol, was assigned by comparing the 13 C NMR data with yojironins C and D ( Mamemura et al., 2011). Thus, the planar structure was defined as shown in Fig. 2 View Fig . The NOESY correlation of H-8/Me- 10 in 2 suggested that H-8 and Me-10 were situated at the same plane. Additionally, it was reported that the chemical shifts of C-10 and C-11 were different in erythro or threo isomers, for example: a pair of epimers carvenosine ( erythro) and epicarvenosine ( threo) ( Jefford et al., 1987; Mamemura et al., 2011). Comparison of the chemical shifts for C-10 ( δ C 23.0) and C-11 ( δ C 37.7) of 2 with those of the corresponding positions in carvenosine ( δ C 23.3 and 36.9, respectively) and epicarvenosine ( δ C 20.6 and 39.5, respectively) implied the erythro relationship for C-8/C- 9 in 2. It was verified by comparing the chemical shifts of C-10 and C-11 with those of yojironin C ( δ C 22.8 and 37.7) ( Mamemura et al., 2011). However, the relative configuration of C-2 ′ could not be assigned due to the far distance between the other two chiral carbons. Furtherly, ECD calculations were carried out for (8 R,9 S, 2 ′ S), (8 S,9 R,2 ′ R), (8 R,9 S,2 ′ R), and (8 S,9 R,2 ′ S)- 2. Results showed that the experimental ECD curve matched well with (8 R,9 S,2 ′ S), as shown in Fig. 3 View Fig . Thus the structure of hyphengshanol B was elucidated as 2.
Hyphengshanol C ( 3) was obtained as a pale yellowish powder. It exhibited a negative ion m / z 361.2004 [M ‒ H] ‒ (calcd for 361.2020) in the HR-ESI-MS spectrum, suggesting the molecular formula C 21 H 30 O 5. Similarly, it also displayed a group of signals of phloroglucinol coupled with a 2-methylbutanoyl group. The 13 C and HSQC data suggested the resonance of an isoprenyl group [ δ H 1.59 (3H, s), δ C 17.8; δ H 1.66 (3H, s), δ C 25.8; δ C 132.4; δ H 5.07 (1H, s), δ C 123.8; δ H 2.09 (2H, m), δ C 21.9] as well as a methyl, two methylenes, an oxygenated methyne, and an oxygenated tertiary carbon ( Table 1). HMBC crosspeaks from H-7 to C-4, C-5, and C-6 and from H-10 to C-8, C-9, and C-11 combined with COSY correlations of H-7/H-8, H-11/H-12, and H-12/H-13 established the monoterpenoid framework as in Fig. 2 View Fig . Compared with the known compound empetrikarinol B, the shielded chemical shift of aromatic proton at 5.76 ppm ( δ H 5.96 in empetrikarinol B) indicated that the 2- methylbutanoyl group was located at C-3.
NOESY correlations of H-8/H-11 and H-8/H-12 are similar to the reported data of empetrikarinol A ( Schmidt et al., 2012a), suggesting the orientation of H-8 and Me-10 as shown in Fig. 2 View Fig . However, the relative configuration of C-2 ′ could not be assigned. Quantum calculations were carried out for the ECD curves of (8 S,9 R,2 ′ S), (8 S,9 R,2 ′ R), (8 R,9 S,2 ′ S), (8 R,9 S,2 ′ R)- 3. The comparison of experimental and calculated ECD curves showed that both (8 R,9 S,2 ′ R) and (8 R,9 S,2 ′ S) are similar to the experimental data ( Fig. 3 View Fig ), indicating that the change in configuration at C-2 ′ has a slight effect on the ECD data. Finally, the structure of hyphengshanol C was assigned as (8 R,9 S)- 3.
Compounds 4 and 5 were obtained as pale yellowish powders. They displayed the same UV, HR-ESI-MS, 1 H NMR, and 13 C NMR spectroscopic data with empetrikarinols A and B, respectively ( Schmidt et al., 2012a). However, compound 4 was optically inactive, suggesting it was a racemate. Resolution via chiral-phase HPLC afforded a pair of enantiomers 4a and 4b with a ratio of 1:1 (Fig. S28). In the 13 C NMR spectrum (Fig. S29) of 5, most of the carbon resonances displayed duplicated peaks, in particular those of the 2-methylbutanoyl group [ δ C 46.0, 46.2 (C-2 ′); 26.6, 27.1 (C-3 ′); 11.9, 12.1 (C-4 ′); 16.7, 17.4 (C-5 ′)], indicating the presence of C-2 ′ epimers. A resolution on 5 was carried out by chiral-phase HPLC, resulting in the isolation of 5a and 5b with a ratio of about 1:1 (Fig. S30). The ROESY spectrum of 5 was similar to empetrikarinol B ( Schmidt et al., 2012a), and correlations of H-8/H-11 and H-8/H-12 suggested H-8 and Me-10 were situated at the opposite plane and discretionarily assigned the orientation of 8 β -H and10 α -Me in 5. Thus relative configurations of 4 and 5 were deduced as the same as empetrikarinols A and B. The absolute configurations were assigned by comparison of experimental and calculated ECD curves, as shown in Fig. 3 View Fig . The experimental ECD curves of 4a and 4b matched well with (8 S,9 R) and (8 R,9 S)- 4, respectively. Based on the negative specific rotation ([ α] D 25 10.0) of 4a, it was identified as ()-empetrikarinol A, and the absolute configuration was defined as (8 S,9 R). And 4b was determined as an undescribed compound with the (8 R,9 S) configuration, named (+)-empetrikarinol A. Similar to the aforementioned 3, the configuration of C-2 ′ in 5 was difficult to be determined by ECD and NMR calculations. Finally, the structures of 5a and 5b were defined as (8 S,9 R)-empetrikarinol B and (8 R,9 S)-empetrikarinol B, respectively.
Compound 6 was identified as 1-[3,4-dihydro-5,7-dihydroxy-2- methyl-2-(4-methyl-3-penten-1-yl)-2H-1-benzopyran-8-yl]-2-methyl-1- propanone by comparing its spectroscopic data with those reported in literature ( Schmidt et al., 2012a). Herein, we determined its (9 R) configuration for the first time, using the ECD calculation method ( Fig. 3 View Fig ).
Hyphengshanol D ( 9), obtained as a pale yellowish powder, gave the molecular formula C 21 H 30 O 5 based on the protonated ion at m / z 363.2167 [M + H] + (calcd for 363.2166) in the HR-ESI-MS spectrum. Its 1 H NMR and 13 C NMR data ( Table 2) were highly similar to those of 11. Different chemical shifts of the aromatic proton at 5.72 ppm in 9 and 5.94 ppm in 11 indicated that the 2-methylbutanoyl group was located at C- 3 in 9. The planar structure was confirmed by HMBC and COSY correlations as depicted in Fig. 2 View Fig . ROESY correlations of H-8/H-13, H-8/ H-7a, and H-10/H-7b suggested the orientation of these substituents as 8 α -H, 10 β -Me, and 13 α -H. Chiral-phase HPLC analysis of 9 afforded a pair of peaks with a ratio of 1:1, indicating that 9 was a pair of isomers. ECD curves of four potential absolute configurations were stimulated, as depicted in Fig. 3 View Fig . However, the configuration C-2 ′ could not be assigned. Compounds 9a and 9b were finally defined as (8 R,9 R,13 R)- hyphengshanol D and (8 S,9 S,13 S)-hyphengshanol D, respectively.
Compounds 15–17 were tentatively identified as hyperjovinol A ( Athanasas et al., 2004), dauphinol F ( Fuentes et al., 2018; Pearce et al., 2021), and empetrikathiforin ( Schmidt et al., 2012b), respectively, by comparing their previously reported UV, HR-ESI-MS, and 1 H NMR and 13 C NMR spectroscopic data. However, analyses by chiral-phase HPLC showed that they were three pairs of isomers with a ratio of about 1:1 (Figs. S48, S51, S54). Compounds 15a and 15b were a pair of enantio-
25 25 mers with specific rotation values [ α] D 16.0 ( c 0.05, MeOH) and [ α] D + 12.0 ( c 0.05, MeOH). The ECD calculation resulted in the absolute configuration of 15a and 15b being elucidated as (9 S) and (9 R), respectively. Compounds 16a and 16b were a pair of epimers with 25 25
specific rotation values [ α] D + 16.0 ( c 0.05, MeOH) and [ α] D + 81.9 ( c 0.05, MeOH). The ECD and specific rotation calculations for four possible stereoisomers of dauphinol F were previously carried out ( Pearce et al., 2021). Dauphinol F corresponded to the (9 R,2 ′ S) configuration according to the positive cotton effect at 290 nm in the ECD spectrum and the specific rotation value of + 18.2 ◦ dm 1 (g/mL) 1. Compound 16a was identified as dauphinol F due to its experimental ECD and specific rotation data being highly similar to those of dauphinol F. The stereoisomer 16b was assigned as (9 S,2 ′ S) based on the positive cotton effect at 290 nm in the ECD spectrum and the specific rotation
25 value of [ α] D + 81.9 (calculated for + 50.2). Similarly, ECD calculations for four possible stereoisomers of empetrikathiforin were carried out in the current study. The stimulated ECD curve of (8 R,2 ′ R)- 17 exhibiting a negative cotton effect at 220 nm and a positive cotton effect at 290 nm matched well with the experimental ECD data of 17a. Additionally, the calculated ECD curve of (8 R,2 ′ S) and experimental ECD curve of 17b both showed positive cotton effects at 220 and 290 nm. Thus, the absolute structures of 17a and 17b were defined as (8 R,2 ′ R) and (8 R,2 ′ S), respectively.
Compound 19 was identified as 3-geranyl-1-(2 ′ -methylbutyryl)- phloroglucinol by comparing their previously reported UV, HR-ESI-MS, 1 H NMR, and 13 C NMR spectroscopic data ( Schmidt et al., 2012b). Although it was reported as a major compound from several Hypericum species, the absolute configuration of C-2 ′ has not been determined for now. A comparison analysis of calculated and experimental ECD data assigned 19 as (2 ′ S)-3-geranyl-1-(2 ′ -methylbutyryl)-phloroglucinol ( Fig. 3 View Fig ).
The other eight known compounds 1-[5,7-dihydroxy-2-methyl-2-(4- methylpent-3-enyl)chroman-6-yl]-2-methylbutan-1-one ( 7) ( Schmidt et al., 2012a), 1-[5,7-dihydroxy-2-methyl-2-(4-methylpent-3-enyl)chroman-6-yl]-2-methylpropan-1-one ( 8) ( Schmidt et al., 2012a), madeleinol A ( 10) ( Fobofou et al., 2015), empetriferdinol ( 11) ( Schmidt et al., 2012a), 3 ′ -methylhyperjovoinol B ( 12) ( Fuentes et al., 2018), empetriferdinan A ( 13) ( Schmidt et al., 2012a), empetriferdinan B ( 14) ( Schmidt et al., 2012a), and 3-geranyl-1-(2 ′ -methylpropanoyl)- phloroglucinol ( 18) ( Schmidt et al., 2012b) were identified by comparing their physiochemical data with those previously reported. These MAPs isolated from H. hengshanense showed a structural diversity. They could be classified as mono- ( 15–19), bi- ( 2–8), tri- ( 9–14), and poly-cyclic acylphloroglucinols ( 1) according to the number of rings in their structures.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.