Sobarocephala icmbio, Rafael & Marques & Limeira-De-Oliveira, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5353.4.2 |
|
publication LSID |
lsid:zoobank.org:pub:16BD810F-4FB7-4D65-995E-1BA6FCBB2788 |
|
DOI |
https://doi.org/10.5281/zenodo.8431288 |
|
persistent identifier |
https://treatment.plazi.org/id/04FEB389-0540-4169-AF76-12C460C998EE |
|
taxon LSID |
lsid:zoobank.org:act:04FEB389-0540-4169-AF76-12C460C998EE |
|
treatment provided by |
Plazi |
|
scientific name |
Sobarocephala icmbio |
| status |
sp. nov. |
Sobarocephala icmbio View in CoL sp. nov.
urn:lsid:zoobank.org:act:
( Figs 41–62 View FIGURES 41–51 View FIGURES 52–61 View FIGURE 62 )
Diagnosis. Face entirely ivory white in the male, with a mid-longitudinal black stripe in the female. Postcranium yellow midlongitudinally, black laterally on the dorsal half, yellow on the ventral half. Thorax of male with scutum mostly yellow; mesopleuron with anepisternum ivory white on anterior half, black on posterior half; katepisternum mainly yellow with central black spot; anepimeron black with anterior margin yellow. Notopleuron, postalar callus, and scutellum ivory white. Mediotergite yellow; laterotergite black. Wing hyaline, slightly brown, infuscated on the distal third along the costal margin and apex. M 1+2 ratio 3.8. Tergites 1–2 ivory white, tergites 3–6 shiny black; tergite 7 yellow, remaining black. Annulus yellow. Epandrium black. Surstylus is narrowly triangular, pointed, and slightly inward curved. The female scutum is mostly black, with a mid-longitudinal black stripe extending from the level of the postpronotal lobe to the scuto-scutellar suture, widening posteriorly.
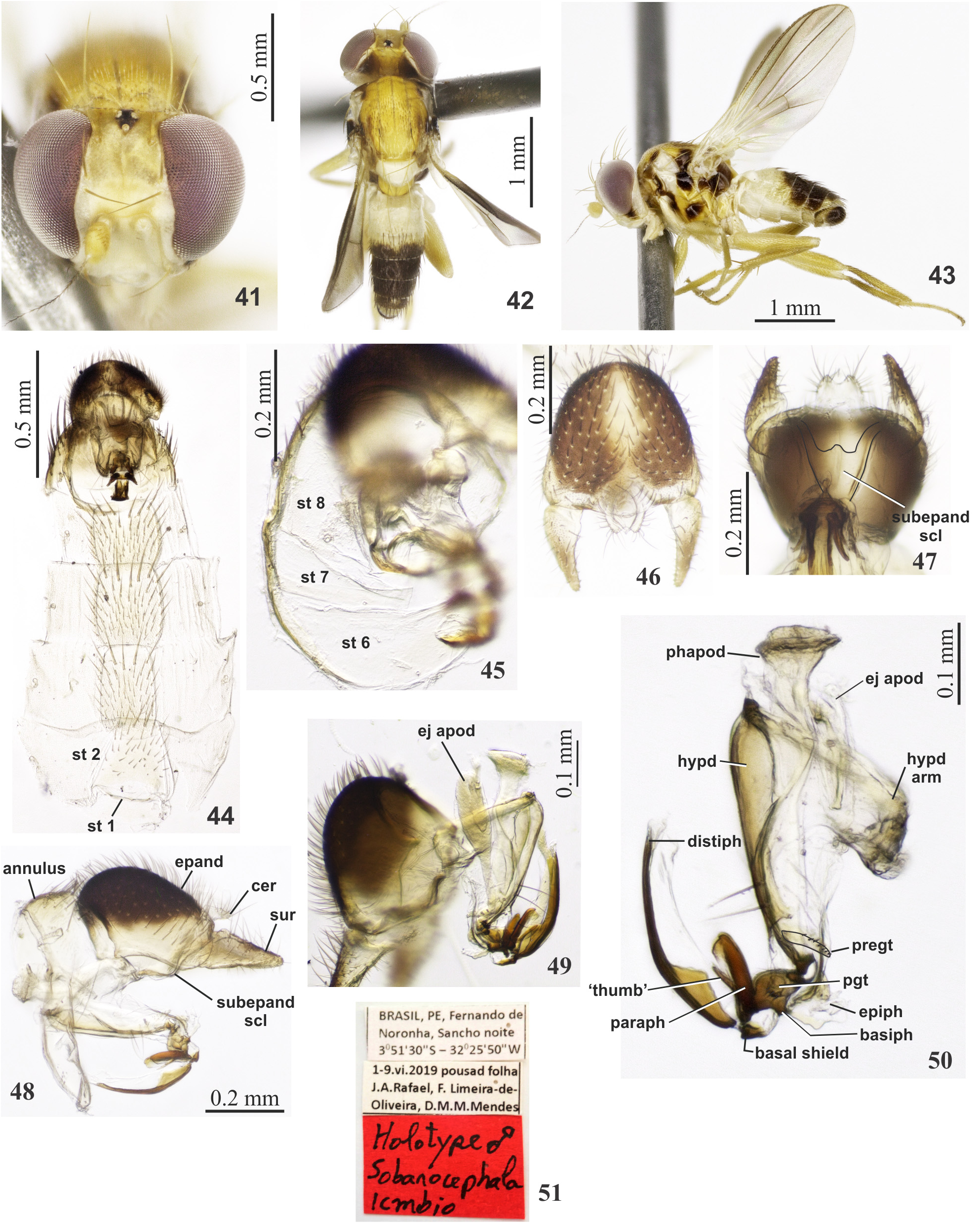
Description. Holotype ♂ ( Figs 41–43 View FIGURES 41–51 ). Body length 3.4 mm; wing length 2.8 mm. Head. Frons light yellow, sides converging dorsally; lunule light yellow white pruinose; ocellar tubercle shiny black ( Fig. 41 View FIGURES 41–51 ). Postcranium ( Fig. 42 View FIGURES 41–51 ) yellow mid-longitudinally, black laterally on dorsal half, yellow on ventral half. Gena ( Fig. 43 View FIGURES 41–51 ) ivory white with vertical black spot. Face ivory white ( Fig. 41 View FIGURES 41–51 ). Antenna yellow, arista short plumose ( Fig. 43 View FIGURES 41–51 ). Thorax. Postpronotal lobe and region internal and posterior to notopleuron brown; scutum mostly shiny yellow, ivory white at the level of postalar callus and on scutellum ( Fig. 42 View FIGURES 41–51 ) with narrow postsutural faded black intra-alar stripe. Notopleuron ivory white anteriorly, black posteriorly. Setae: acrostichal multiseriate. One pair of lateral scutellar plus two pairs of shorter subapical, lateral setae. Subscutellum and mediotergite yellow. Laterotergite shiny black ( Fig. 43 View FIGURES 41–51 ). Anepisternum ivory white on anterior half, black on posterior half; katepisternum mainly yellow with central black spot; anepimeron black with anterior margin yellow; meron and metasternum yellow. Legs light yellow with coxae lighter ( Fig. 43 View FIGURES 41–51 ). Wing hyaline, slightly brown infuscated on distal third along costal margin and apex ( Fig. 43 View FIGURES 41–51 ). M 1+2 ratio 3.8. Abdomen. Tergites 1–2 ivory white, tergites 3–6 shiny black; tergite 7 yellow, remaining black ( Figs 42–43 View FIGURES 41–51 ). Sternites ( Fig. 44 View FIGURES 41–51 ) yellow; sternite 1 reduced to narrow band, weakly sclerotized sternites 2 to 5 complete. Sternite 6 and 7 translucent; sternite 8 dorsal, setose ( Fig. 45 View FIGURES 41–51 ). Terminalia. Annulus yellow. Epandrium black dorsally, paler mid-longitudinally, and yellow at ventral and posterior margins ( Figs 46–47 View FIGURES 41–51 ). Surstylus narrowly triangular, in dorsal view, slightly inward curved, and pointed ( Fig. 46 View FIGURES 41–51 ). Cercus is projecting and distinctly C-shaped, inward curved, with one longer distal seta. Subepandrial sclerite V-shaped, both arms with median translucent connection ( Fig. 47 View FIGURES 41–51 ). Hypandrial arm weakly sclerotized; hypandrium more sclerotized, with two distinct subapical setae on each side ( Fig. 49 View FIGURES 41–51 ). Phallapodeme rod-like, slightly sinuose ( Figs 48–50 View FIGURES 41–51 ). Ejaculatory apodeme somewhat membranous, with elongated sperm pump ( Fig. 49 View FIGURES 41–51 ). Pregonite small, flat, translucent with 5–6 small setae; postgonite small, translucent, subspherical, with 3–4 small marginal setae; epiphallus translucent; basiphallus small, semi-lunar, concave-convex; basal shield of distiphallus with distinct lateral lobe (paraphallus) with thin needle-shaped lateral thumb; distiphallus slightly curved and three-fourth length of phallapodeme ( Figs 49–50 View FIGURES 41–51 ).
Female. As described for males except as follows: Face ivory white with mid-longitudinal black stripe ( Fig. 52 View FIGURES 52–61 ). Scutum mainly black with wide mid-longitudinal black stripe widening posteriorly, extending from level of postpronotal lobe to scutum-scutellar suture ( Fig. 53 View FIGURES 52–61 ). Subscutellum black. Katepisternum, anepimeron, and meron are entirely black ( Fig. 54 View FIGURES 52–61 ). Legs with distal tarsomeres are light brown ( Fig. 54 View FIGURES 52–61 ). Tergites 1 and 2 are ivory white, 4–7 black, remaining yellow. Basal sternites yellow ( Fig. 55 View FIGURES 52–61 ), sternite 1 reduced, sternites 2–4 rectangular, longer than wider, setose. Sternite 6 brown, narrow, 8X longer than wide ( Fig. 56 View FIGURES 52–61 ). Sternite 7 split, basal piece triangular with a pair of short basal setae; posterior piece rectangular, somewhat inconspicuous, with a pair of longer apical setae ( Figs 56–57 View FIGURES 52–61 ). Sternite 8 distinctly bilobed ( Fig. 58 View FIGURES 52–61 ). Sternite 10 with the apex upward directed ( Fig. 57 View FIGURES 52–61 ). Spermatheca spherical ( Fig. 59 View FIGURES 52–61 ).
Eggs. ( Figs 60–61 View FIGURES 52–61 ). Length 0.4 mm, width 0.1 mm, translucent, unpigmented with chorion undulating (outlined in Fig. 61 View FIGURES 52–61 ). Average of 71 eggs within each abdomen, varying from 51 to 93 eggs in five female specimens.
Variation. Male. Body length varies from 3.0– 3.7 mm (n=10); some paratypes with tergite 3 yellow at the basal margin; scutum with paramedian black stripe interrupted between the level of anterior and posterior dorsocentral setae. Female. The body length varies from 3.2–4.7 mm (n=15).
Material examined. BRASIL,PE[= Pernambuco],Fernando de Noronha,Sancho noite, 3°51’30”S – 32°25’50”W / 1–9.vi.2019, pousada na superfície abaxial da folha, J.A. Rafael, F. Limeira-de-Oliveira, D.M.M. Mendes / Holotype ♂ Sobarocephala icmbio ( Fig. 51 View FIGURES 41–51 ) ( INPA). Paratypes pinned: idem (12♂, 6♀ INPA); idem, Trilha Sancho, 1– 9.vi.2019, Malaise (1♂, 3♀ INPA); idem, 1–9.vi.2019, Shannon ( 1♀ INPA); idem, 3°51’17”S – 32°26’26”W, Trilha Sancho, 1–9.vi.2019, Malaise Pq.[=Pequena] (1♂ INPA); idem, 20–27.ii.2020, Shannon, J.A. Rafael, P.C. Grossi, F. Limeira-de-Oliveira ( 1♀ INPA); idem, Sancho, 1–9.vi.2019, Malaise Pq., J.A. Rafael, F. Limeira-de-Oliveira (1♂, 4♀ INPA); idem, 9–24.vi.2019, Malaise Pq., J.A. Rafael, F. Limeira-de-Oliveira, L.C. Castro ( 13♀ INPA); idem, Malaise G ( 1♀ CZMA); idem, 23.vii–7.viii.2019, Malaise Pq ( 4♀ CZMA); idem, 7–21.viii.2019 (2♂, 2♀ CZMA); idem, 8–27.x.2019 (1♂ MNRJ); idem, 9–27.xii.2019, (1♂ MNRJ); idem, 23.i–12.ii.2020 ( 6♀ MNRJ); idem, 12– 27.ii.2020 ( 6♀ INPA); idem, 9–27.xii.2019 ( 1♀ INPA); idem, Capim-Açu, 9–24.vi.2019, Malaise Gd ( 14♀ INPA); idem, 8–25.ix.2019 (1♂, 2♀ MNRJ); idem, 11–27.xi.2019 ( 1♀ MZSP) idem, Trilha Atalaia, 20–27.ii.2020, Malaise Pq, P.C. Grossi, F. Limeira-de-Oliveira (1♂ MZSP); idem, Baia Sueste, 3°51’30”S – 32°25’50”W, 1–9.vi.2019, Arm.[=Armadilha] Luminosa, J.A. Rafael, F. Limeira-de-Oliveira, D.M.M. Mendes (1♂ MZSP); idem, Sueste mangue, Malaise ( 1♀ MZSP); Tr. Golfinhos, 3°51’17”S – 32°26’26”W / 5–20.viii.2019, Malaise Gd, J.A. Rafael, F. Limeira-de-Oliveira, L.C. Castro (3♂, 4♀ CZMA).
Paratypes in alcohol. BRASIL, PE[= Pernambuco], Fernando de Noronha, 3°51’17”S – 32°26’26”W, Capim-Açu, 9–24.vi.2019, Malaise G, J.A. Rafael, F. Limeira-de-Oliveira, L.C. Castro ( 13♀ INPA) GoogleMaps ; idem, 8–23.vii.2019, ( 9♀ INPA) GoogleMaps ; idem, 23.vii–7.viii.2019 ( 4♀ INPA) GoogleMaps ; idem, 7–21.viii.2019 (1♂, 2♀ INPA) GoogleMaps ; idem, 21.viii–8.ix.2019 (3♂, 1♀ MZSP) GoogleMaps ; idem, 25.ix–8.x.2019 (2♂ MNRJ) GoogleMaps ; idem, 8–27.x.2019 (2♂ INPA) GoogleMaps ; idem, 11–27.xi.2019 (6♂ INPA) GoogleMaps ; idem, 10–23.i.2020 ( 1♀ INPA) GoogleMaps ; idem, Sancho , 9–24.vi.2019, Malaise Pq, J.A. Rafael, F. Limeira-de-Oliveira, L.C. Castro ( 2♀ INPA) GoogleMaps ; idem, 24.vi–8.vii.2019 ( 5♀ INPA) GoogleMaps ; idem, 8–23.vii.2019 ( 4♀ INPA) GoogleMaps ; idem, 27.xii–10.i.2020 ( 3♀ INPA) GoogleMaps ; idem, 10–23.i.2020 ( 2♀ INPA) GoogleMaps .
Holotype condition. Good, pinned, and mounted on card point, not dissected.
Etymology. The specific epithet is a noun in apposition in honor of the Brazilian Instituto Chico Mendes de Conservaç ã o da Biodiversidade (ICMBio), which develops programs, actions, and projects seeking the conservation of natural resources to improve the quality of life of this and future generations.
Remarks. The specimens from Fernando de Noronha key to S. atrifacies Sabrosky & Steyskal, 1974 in Lonsdale & Marshall (2007b) and Lonsdale & Marshall (2012). Unfortunately, the male of S. atrifacies is unknown for comparison. The female of S. icmbio sp. nov. differs from S. atrifacies by having a black postpronotal lobe (versus yellow, sometimes black); a scutum with a black spot adjacent to the postpronotal lobe (versus black spot absent); a scutum with a mid-longitudinal black stripe extending from the level of the postpronotal lobe to the scuto-scutellar suture that widens posteriorly (versus scutum with large squared posteromedial black spot); mesopleuron with black stripe and spots [mesopleuron white and yellow in S. atrifacies ] and tergites 3–6 entirely black (versus tergites 3 and 5 yellow anterolaterally).
Sobarocephala icmbio sp. nov. belongs to the Sobarocephala flaviseta species group, and within this species group, it is allied to those species best characterized by white spots on the notopleuron, scutellum, and postalar callus; the Sobarocephala flaviseta species group are mostly Central American in distribution, but some extend into mostly the northern parts of tropical South America.
Bionomics. Around 140 adult specimens of Sobarocephala icmbio sp. nov. were collected using flight interception traps with a male: female ratio of 1:3. The skewed sex ratio was probably a result of males spending more time perched on leaf surfaces to hold and patrol their territory or to ward off males vying for lek sites to attract females. Sexes ratio occurred near equal proportion on the Capim-Açu and Sancho trails. The clusiids are active during the day, and some use characteristic substrates such as dead wood, as well as other prominent surfaces, including large fallen leaves ( Marshall 2000; Roháček 1995; Lonsdale & Marshall 2006 b), as lek sites where males hold, and patrol territory to attract females, who evaluate the males, at least partially, on the quality of the territory held ( Lonsdale & Marshall 2012). This way, males remain perched for longer periods, while females actively fly to seek lek sites. Consequently, females are more susceptible to being captured in flight interception traps. Within Sobarocephala , observations potentially consistent with lekking have been made for S. daidaleos Lonsdale & Marshall, 2012 in Bolivia, where males were found patrolling patches of territory on the underside of broad leaves. We also found two lek sites at night on the underside of the same plant. We observed agglomerations of seven male and five female specimens on one leaf ( Fig. 62 View FIGURE 62 ), with a smaller number found on two other leaves of the same unidentified plant species. Lek sites were apparently inactive during the night, specifically around 9 pm on June 6, 2019. During this time, when the specimens were slightly disturbed, they moved a little bit but remained on the same leaf. This is the first observation of an agglomeration during nighttime.
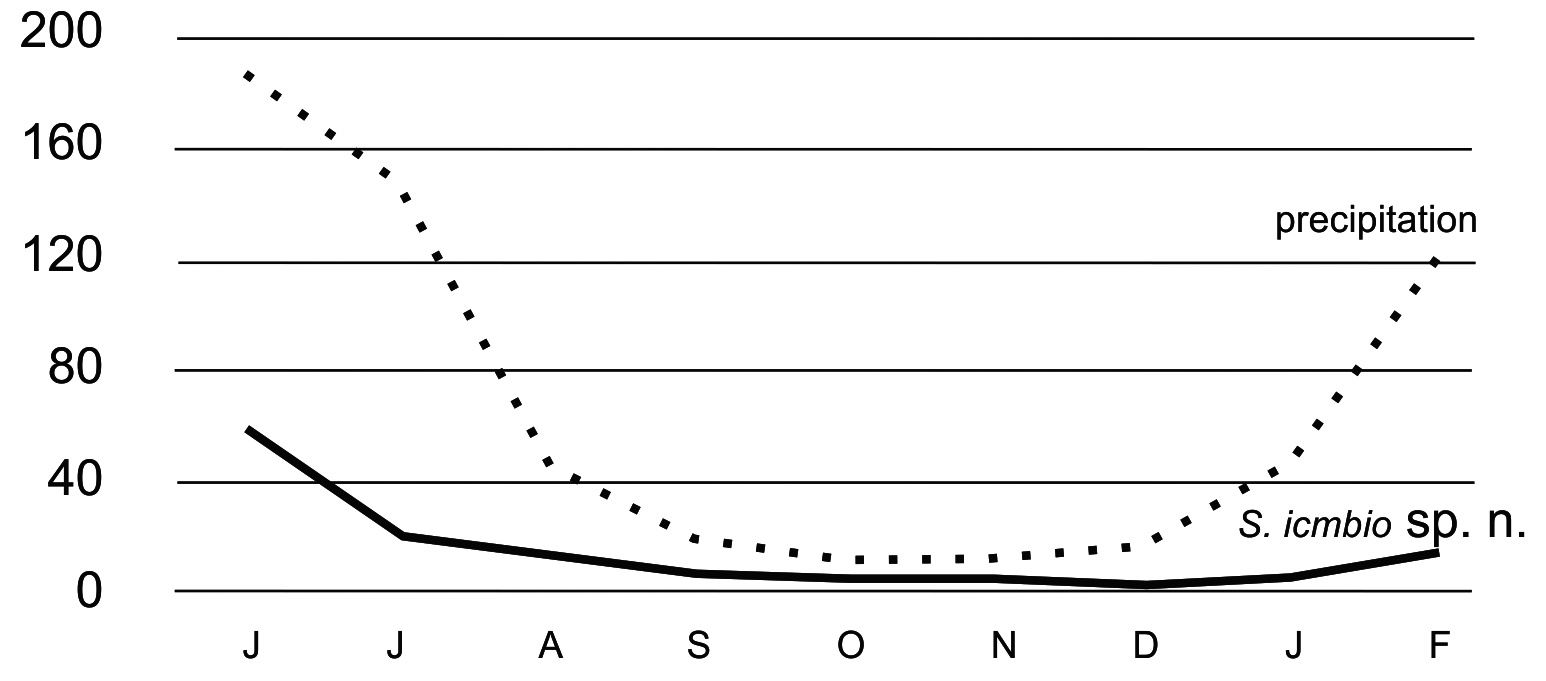
The specimens were collected from June to February using flight interception traps. The highest density of adults was at the beginning of collections in June, which coincided with the end of the rainy season in the archipelago. Another peak in adult density was observed during the last month of collection in February, when precipitation levels began to increase again. Unfortunately, the collections were interrupted in March/2020 due to the covid-19 pandemic, and the apparent trend of increasing population density could not be further verified. The seasonal occurrence analysis ( Fig. 63 View FIGURE 63 ) revealed a positive linear relationship with precipitation (Pearson r = 0,845, p<0.05). Based on this correlation, we can predict that there will be more specimens in the following three months (March, April, and May).
The only description of a Sobarocephala egg is from S. uberis Lonsdale & Marshall, 2012 ( Lonsdale & Marshall, 2012) based on a single egg. We examined five females and reached an average of 71 eggs in the females with internal eggs. This finding was interesting and provided valuable information, suggesting that this quantity of eggs could be considered significant for the Sobarocephala species’ maintenance in the archipelago.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |