Gymnospermium maloi, Kit Tan & Shuka, 2011
|
publication ID |
https://doi.org/10.11646/phytotaxa.25.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/945D8788-FFED-FE58-2C8B-5F177B05612C |
|
treatment provided by |
Felipe |
|
scientific name |
Gymnospermium maloi |
| status |
|
Biology of Gymnospermium maloi View in CoL and G. scipetarum
The tubers of Gymnospermium maloi are positioned 15–20 cm below the ground depending on soil depth. The vegetative buds are formed in a depression on the upper part of the tuber. They begin their development soon after the first autumn rains. The young shoots elongate slowly underground and attain a length of 4–6 cm by late December and up to 10 cm by late January. The flowering stems emerge above ground in early March, before the basal leaves. Flowering begins soon after in mid-March, extending to mid-April or May depending on elevation. This can be compared to the situation in G. scipetarum where the basal leaves develop and emerge before the flowering stems, very rarely appearing at the same time.
In its native habitat Gymnospermium maloi usually flowers 10–15 days before G. scipetarum . However, in cultivation it comes into flower by late January and the first half of February and there is only a one week gap between the two species. Both species were observed to be actively visited by honeybees ( Apis mellifera ) and bumblebees ( Bombus terrestris ). In Curtis’s Botanical Magazine ( McNab 1833), there is an illustration of G. altaica with straight filaments, but the stamens are actually slightly zig-zag, with the lower part of the filament fitting into the nectar-producing hollow or pocket at the base of the petal, and the upper antherbearing half is excurved above the petal ( Fig. 1 A View FIGURE 1 3 & B3 View FIGURE 3 ). This feature is not easily perceived in herbarium material as the stamens often appear “straight” after drying.
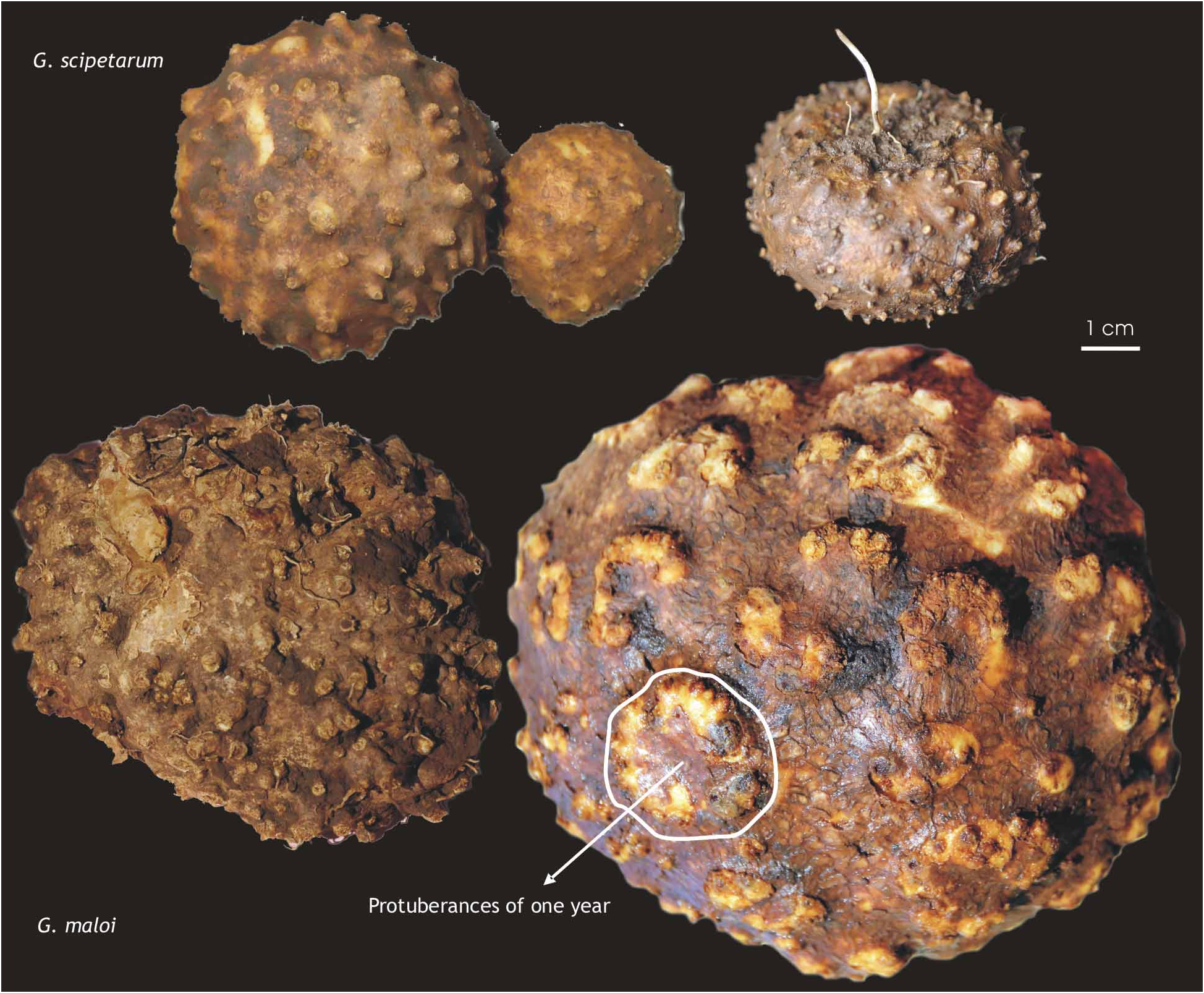
The seeds mature in May and June and remain attached for a short time before dispersal. The aerial parts die down by mid-July, and the tubers lie dormant until the rains in October. Both species are easily grown from tubers; plants of G. scipetarum have survived at Copenhagen for more than 10 years, flowering and fruiting annually. The position of the previous year’s flowering stem and basal leaves is each marked on the tuber by a wart-like protuberance the following year ( Fig. 4 View FIGURE 4 ). The mean of 100 plants and c. 60 tubers were used for our calculations to estimate how old the plants were. The number of protuberances on the tubers dug up (150–400 for G. maloi and 50–270 for G. scipetarum ) indicate that plants can be 13–30 years or more and in one case we have calculated an age of 40 years. The tubers of G. maloi at flowering weigh 150–650 g with the oldest tubers being also the largest and heaviest. The tubers of G. scipetarum are smaller and weigh 40– 280 g.
The affinities of G. maloi lie clearly with G. scipetarum . It differs from the latter by its much larger tubers, more numerous basal leaves and flowering stems as well as number of flowers per raceme. It can also be distinguished by its thicker and more glaucous leaflets. The plants of G. peloponnesiacum always remain low in stature and small in vegetative and floral parts ( Fig. 6 View FIGURE 6 ). Selected morphological characters of the three Balkan species are summarized in Table 1.
In addition to morphological differences between the species, there are also differences in the type of habitat and soil composition. The Quercus-Ostrya forest habitat of G. maloi and Abies cephalonica forest habitat of G. peloponnesiacum both overlie hard limestone. Limestone overlying flysch predominates in the habitat of G. scipetarum . Differences in soil composition are observed for the two Albanian species ( Table 2). The amounts of soluble exchangeable Ca 2+, Mg 2+ and heavy metals Mn 2+ and Ni 2+ differ greatly in the substrates of G. maloi and G. scipetarum . For G. scipetarum , despite a higher Total Organic Carbon content (TOC) of 35.46 % and higher water-holding capacity in the black soil of shady Fagus forest where it occurs, the tubers are only half the size of those of G. maloi . The red soil in habitats of G. maloi provides less nutrient and organic matter (lower TOC of 27.8%), and has a lower water-holding capacity but the tubers become very large. The disparate link between rich organic soil composition and small tuber size can be compared to the positive correlation between high number of basal leaves and increased LAI values ( Tables 1 & 2). A Leaf Area Index (LAI) of 0.7–1.4 m 2 m -2 and 0.4–0.8 m 2 m -2 for G. maloi and G. scipetarum respectively was calculated.
In order to avoid the hot and dry summer months, vegetative growth occurs only during early spring. The populations of G. maloi inhabit East-, Northeast- or Southeast-facing slopes in their more xerophytic habitats, whereas G. scipetarum inhabits West- or Northwest-facing slopes in their more damp, moist and humus-rich habitats. An analysis of the floristic composition during the flowering period of all three species reveals that the following genera are often found together with species of Gymnospermium . These are Anemone , Corydalis , Crocus , Galanthus , Geranium , Ranunculus , Teucrium and Viola .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.