Bairdoppilata Coryell, Sample and Jennings, 1935
|
publication ID |
https://doi.org/10.11646/zootaxa.4059.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:3B0DAB43-FB07-4971-B3C5-F2005F7EE67A |
|
DOI |
https://doi.org/10.5281/zenodo.6096172 |
|
persistent identifier |
https://treatment.plazi.org/id/943887AA-FFF4-FA43-239D-F9A56AD38753 |
|
treatment provided by |
Plazi |
|
scientific name |
Bairdoppilata Coryell, Sample and Jennings, 1935 |
| status |
|
Genus Bairdoppilata Coryell, Sample and Jennings, 1935 View in CoL
1935 Bairdoppilata Coryell, Sample and Jennings : 3.
1969 Bairdoppilata (Bairdoppilata) Coryell, Sample and Jennings—Maddocks : 66. 1995 Bairdoppilata Coryell, Sample and Jennings—Maddocks : 215.
History. More than 100 nominal species have been classified in Bairdoppilata ( Kempf 1986, 1995, 2004). Carapaces are recognizable by the accessory (bairdoppilatan) locking dentition, and the living animals have two scissors-like, terminal antennal claws. Because fossil representatives, including the Miocene type species, have been identified chiefly by the supplemental dentition, there was some doubt about the value of the genus for several decades ( Morkhoven 1958, 1963). Confusion arose because supplemental dentition is also well expressed in Glyptobairdia coronata . Shaver (1961, p. 205, fig. 140) recognized Bairdoppilata in the Treatise, but Glyptobairdia was treated as a synonym of Triebelina . The taxonomic history of living ornate bairdiids has been reviewed by Bold (1974), Malz & Lord (1988), Maddocks & Wouters (1990), and many others cited therein. Bolz (1969, 1971) reviewed the prevalence of auxiliary dentition in Triassic genera of Bairdioidea . He was the first to explain its functional significance as a locking mechanism to reduce torque in high-arched carapaces, which increases the likelihood that this character may be subject to convergence. He emphasized the importance of considering all morphological features in a taxonomic diagnosis, rather than just one key character: “No single morphological feature seems alone sufficient to establish higher systematic units” ( Bolz 1971, p. 725).
Brady (1880, Pl. 3, figs. 2b, 3a) illustrated the two distal antennal claws and seven furcal setae of B. villosa , remarking on the prominent barbs of seta 2. Tressler (1949, Figs. 5 View FIGURE 5 , 8 View FIGURE 8 ) illustrated the furca and antenna of B. cushmani but did not mention the two distal claws. Rome (1960) provided a meticulous description of the soft parts of G. coronata , which was then classified in Triebelina , but did not evaluate its distinctive features. It was Kornicker (1961, examining B. carinata , a synonym of B. cushmani ) who pointed out distinctive attributes of the furca and hemipenis that might support a generic diagnosis, although he did not mention the antenna. Kornicker was also the first to realize the taxonomic significance of the patch pattern of the carapace, which is often preserved in fossils. Maddocks (1969) proposed an expanded concept for the genus Bairdoppilata , which incorporated these and other characters of the soft parts and carapace, and which has proved to be sufficiently flexible to accommodate other new species as they turned up.
Species included. The genus Bairdoppilata in its broadest usage includes at least three ecological groups of living species. The most diverse cluster consists of relatively small, mostly punctate species in tropical reef and phytal assemblages. The soft parts have been illustrated (at least in part) for the following shallow-water species of Bairdoppilata (listed alphabetically by original binomen):
Bairdoppilata (Bairdoppilata) alcyonicola Maddocks, 1969 View in CoL ( Nosy Be, Madagascar)
Bairdoppilata angolensis Hartmann, 1974 ( Angola) View in CoL [The published range (minimum to maximum) for carapace length is 30 µm, but for height the range is 110 µm. This is probably an error.]
Bairdoppilata balihaiensis Hartmann, 1978 View in CoL (Northwest Australia)
Bairdoppilata (Bairdoppilata) cratericola Maddocks, 1969 View in CoL ( Nosy Be, Madagascar)
Nesidea cushmani Tressler, 1949 View in CoL (Florida, Bahamas) [= Bairdoppilata carinata Kornicker, 1961 View in CoL by Maddocks 1969]
Bairdoppilata cytheraeformis Hartmann, 1974 ( Angola) View in CoL
Bairdoppilata geelongensis Hartmann, 1980 View in CoL (South Australia)
Bairdoppilata mocamedesensis Hartmann, 1974 ( Angola)
Bairdoppilata portsamsonensis Hartmann, 1978 View in CoL (Northwest Australia) [The identity of this species is in doubt, because more than one species and genus are represented among the published illustrations. The RV belongs to a species of Bairdoppilata View in CoL ( Hartmann 1978, Figs. 13 View FIGURE 13 , 24), but Fig. 14 View FIGURE 14 shows a LV of Neonesidea View in CoL with caudal setae, and Fig. 25 shows a LV of Paranesidea View in CoL with shield-shaped patch pattern. It is obvious that this RV could not fit inside either LV. Hartmann compared the hemipenis to that of B. balihaiensis View in CoL , but his Fig. 31 shows many differences. The long copulatory tube ending in a tight coil is more appropriate for Neonesidea View in CoL .]
Bairdia simuvillosa Swain, 1967 View in CoL (reported from the Gulf of California) [The published illustrations of appendages are from a living female specimen with scissors-like antennal claws, collected in Scammon Lagoon on the Pacific side of Baja California. It was illustrated as Fig. 32a–i of Swain (1967) but probably drawn by Kenneth G. McKenzie. The published illustrations of the valves do not belong to Bairdoppilata View in CoL but to species of Neonesidea View in CoL (Swain, 1967, Figs. 30c, d; Plate I, figs. 2a–f, 8). In a separate paper, McKenzie and Swain (1967, Pl. 30, fig. 1) reported Bairdia simuvillosa View in CoL in Scammon Lagoon, providing a photograph and more plausible carapace dimensions, but they did not mention whether it has bairdoppilatan dentition.]
Bairdoppilata sinusaquilensis Hartmann, 1979 View in CoL (Southwest Australia, also reported by Hartmann (1980) from South and Southeast Australia)
Bairdoppilata View in CoL sp. 2 of Maddocks, 1969 (Northwest Madagascar near Nosy Be)
Bairdoppilata View in CoL ? sp. 2 of Maddocks, 1975 ( Ascension Island)
A second species-group of? Bairdoppilata View in CoL has been reported from sediment samples in deeper and colder water. The carapace is large and nearly smooth. The accessory dentition of the hinge is developed in some populations but inconspicuous or undeveloped in others. Although Maddocks (1969, 1995) suggested that perhaps these species should eventually be classified in a new genus, it would be difficult to diagnose the genus on the basis of present knowledge. The species-level taxonomy is confused. In several cases the anatomical information is taken from doubtfully identified specimens collected at a great distance from the type locality, published at a time when taxonomists were inclined to underestimate taxonomic diversity in the deep sea. Brandão (2008) reviewed the complex history and numerous misidentifications of several of these species in the Southern Ocean and provided important new anatomical, taxonomic and zoogeographic information. The soft anatomy has been described, at least in part, for the following nominal species (listed by original binomen):
Bairdia simplex Brady, 1880 View in CoL (Challenger station 151, off Heard Island, Southern Ocean) [The appendage descriptions by Maddocks (1969) apply to specimen USNM 121347 from Eltanin station 418, near the Antarctic Peninsula, and specimen USNM 121348 from Eltanin station 1345, in the Pacific sector of the Southern Ocean. The identifications of those specimens require verification.]
Nesidea labiata Müller, 1908 View in CoL (Gauss Station, Southern Ocean)
? Bairdoppilata View in CoL sp. 1 aff? B. labiata View in CoL of Brandão 2008 (Southern Ocean)
? Bairdoppilata View in CoL sp. 2 aff? B. labiata View in CoL of Brandão 2008 (Weddell Sea)
Bairdia hirsuta Brady, 1880 View in CoL (Challenger station 300, near Juan Fernandez Island in the Southeast Pacific Ocean) [The appendage descriptions by Maddocks (1969, 1973) apply to specimen USNM 121353 from the Gulf of Mexico and specimen USNM 139891 from Eltanin station 25, near the Galapagos Islands. The identifications of those specimens require verification.]
Bairdia villosa Brady, 1880 View in CoL (Challenger station 149, off Kerguelen Island, Southern Ocean) [The appendage descriptions by Maddocks (1969) apply to specimen USNM 121344 from Eltanin station 418 near the Antarctic Peninsula. That identification requires verification.]
? Bairdoppilata View in CoL sp. 5 of Brandão, 2008 (Knysna Beach, South Africa)
? Bairdoppilata View in CoL sp. 6 of Brandão, 2008 (Lüderitz Bay, Namibia)
A third cluster is represented by the genus Glyptobairdia View in CoL , a small group of Neotropical reef-dwelling species with asymmetrical carapaces, pronounced ridges, deep punctae, bairdoppilatan accessory dentition, and scissors-like antennal claws. The soft anatomy has been described, at least in part, for the following species (listed by original binomen):
Bairdia coronata Brady, 1870 View in CoL (Caribbean, Bermuda, Bahamas, Belize) Bairdoppilata View in CoL ? sp. 1 of Maddocks, 1975 ( Ascension Island; a juvenile)
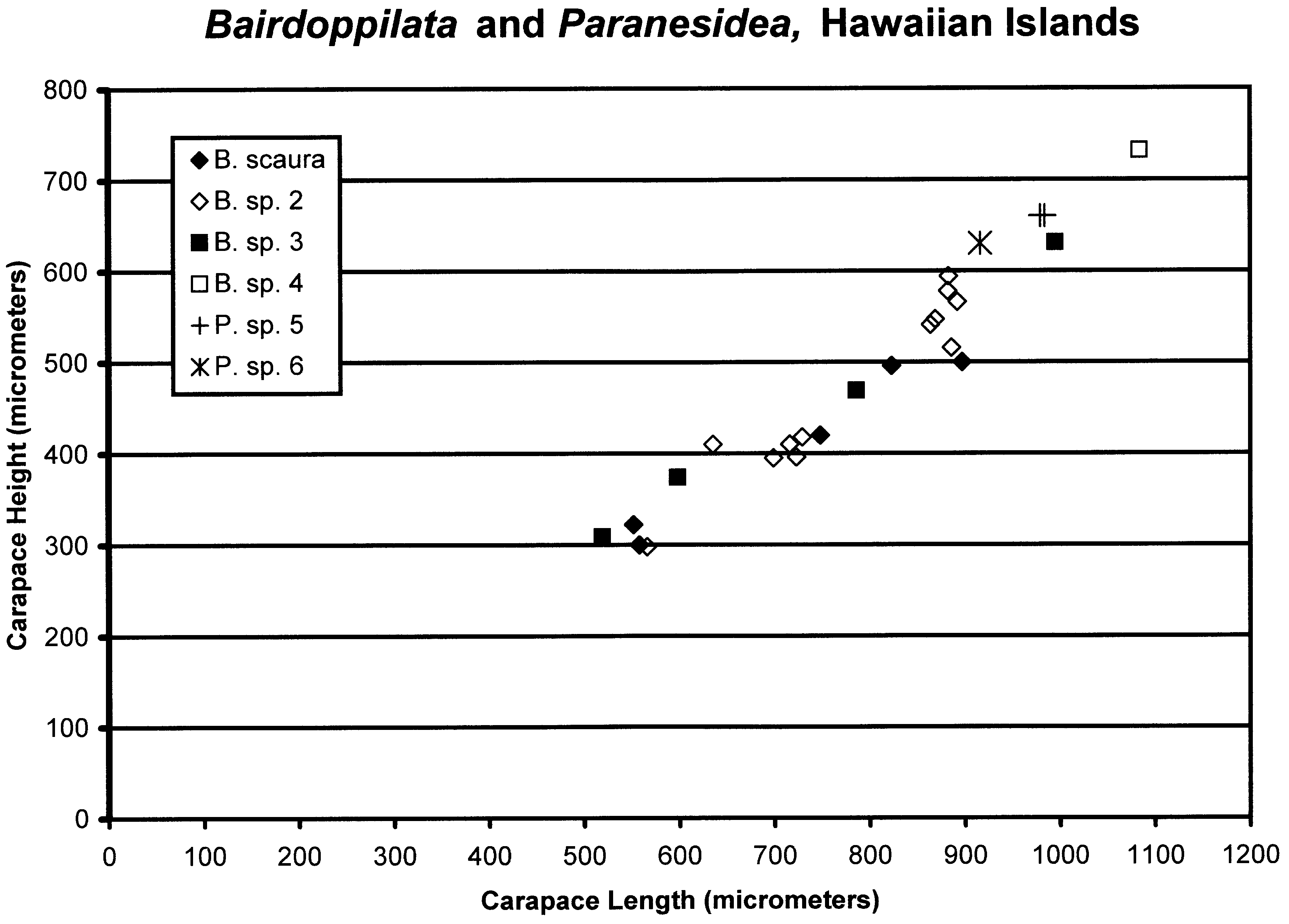
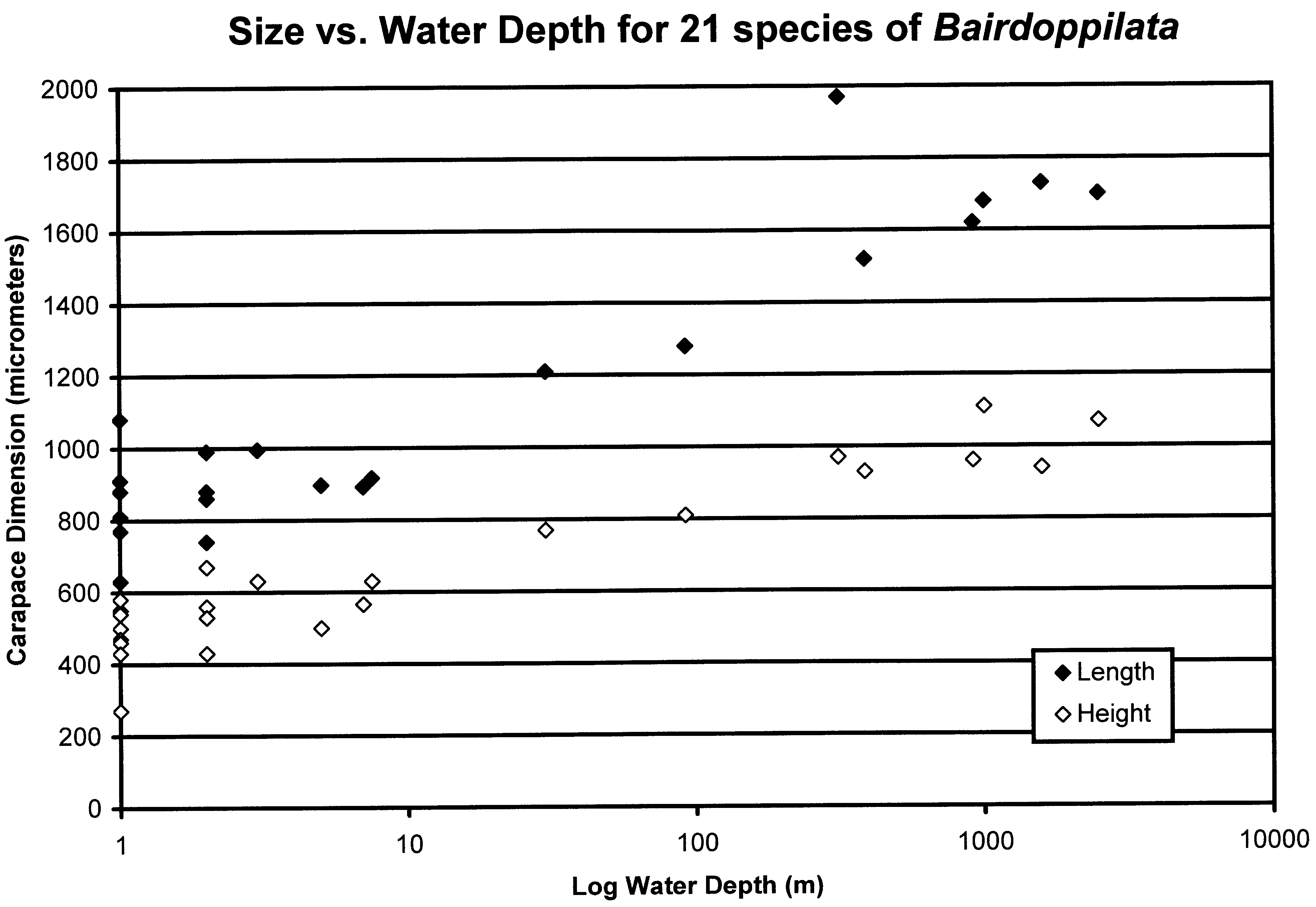
Carapace size and shape. Carapace lengths of species of Bairdoppilata View in CoL range from 0.5 mm ( B. sp. 2, Ascension Island) to almost 2 mm (?B. sp. 1 aff. B. labiata, Antarctic View in CoL Peninsula) ( Table 2, Fig. 3 View FIGURE 3 ). There is a positive association between carapace length and water depth, which involves an inverse relationship with water temperature and latitude. Larger species have been collected from bathyal depths and Antarctic waters. Few species of intermediate size are included in the dataset analyzed here, which is restricted to species whose soft anatomy has been described. The smallest species are those living in intertidal and shallow-subtidal, algal, sandy and coralline habitats in the tropical belt ( Fig. 4 View FIGURE 4 ). Unfortunately, for many of these species the dimensions have been reported only as population ranges (minimum to maximum), which obscures the biological trends.
The carapace height:length proportion ranges from 0.5 to 0.8 ( Table 2) and shows no effect of water depth or latitude. The greatest height is located at 0.47 to 0.51 of length. The carapace thickness:length proportion ranges from 0.41 to 0.48, and the location of greatest thickness is at 0.46 to 0.5 of length. Males are slightly shorter than and not as high as females, although the two populations overlap. This is the usual trend in Bairdiidae View in CoL .
The carapace has conspicuous left-right asymmetry in size and shape, with a larger LV that swells dorsally above the smaller RV. This is because the functional hinge must remain a straight line, independent of the carapace inflation and curvature. The higher the dorsal arch of the LV, the greater the difference in height and outline between the two valves. The LV reaches over the edge of the RV anterodorsally and posterodorsally, as well as ventromedially (as the bow-shaped process behind the mouth region). The lateral outline of the LV is rounded and more distinctive taxonomically, while that of the RV is angulate and less diagnostic. In dorsal view the carapace is moderately compressed, with tapered anterior and posterior ends and gently swollen midsection. The ventral region Explanation of abbreviations: NO = identification number in Figure 3 View FIGURE 3 , NAME = species name or informal identification, REF = published reference, LOC = collecting locality; LCAR = carapace length (Μm), HCAR = carapace height (Μm), H/L = carapace height:length ratio, DEPTH = water depth (m).
sp1 aff lab =? Bairdoppilata View in CoL sp. 1 aff.? B. labiata View in CoL , sp2 aff lab =?B. sp. 2 aff? B. labiata View in CoL , sp. 2 = B. sp. 2 (this paper), sp. 3 = B. sp. 3 (this paper), sp. 4 = B. sp. 4 (this paper).
B2008 = Brandão 2008, H1974 = Hartmann 1974, H1978 = Hartmann 1978, H 1979 = Hartmann 1979, H 1980 = Hartmann 1980, M1969 = Maddocks 1969, M1973 = Maddocks 1973, M1975 = Maddocks 1975, R1960 = Rome 1960, herein = this paper.
ASC = Ascension Island; BAH = Bahama Islands; E25 = Eltanin 25, 0 4o 53’N, 80o28’W to 0 4o 51’N, 80o28’W, east of Galapagos Islands; E418 = Eltanin 418, 62o39–40’S, 56o8–10’W, Antarctic Peninsula; E1345 = Eltanin 1345, 54o50– 51’S, 129o46–48’W, Pacific Sector of Southern Ocean; E1418 = Eltanin 1418, 54o32’S, 159o02’E, Kerguelen Island; FFS = French Frigate Shoals, the Hawaiian Islands; GAUS = Gauss-Station, Antarctica; GM = Gulf of Mexico; KB = Kane’ohe Bay, the Hawaiian Islands; NB = Nosy Be, Madagascar; SB = St. Barthelmy Island, Lesser Antilles; SE AUS = Southeast Australia; W AFR = West Africa; W AUS = West Australia; WED = Weddell Sea, Antarctica.
is not flattened, and the greatest thickness is located a little below mid-height. The exterior surface ranges from smooth to punctate. The preserved valves of shallow-water species are mostly transparent, except for an oval opaque patch located centrally over the adductor muscle scar pattern. There may be brown pigmentation either in two small spots or over much of the lateral surface.
For shallow-water species, the lateral silhouettes of the carapace or LV can be sorted into three intergradational groups, which merely represent combinations of two trends, dorsal inflation and caudal extension:
(1) Oblong, loaf-shaped or scoop-shaped, with low-arched, subtly angulate dorsal margin and smoothly rounded posterior margin, not caudate: B. scaura View in CoL n. sp., B. cytheraeformis View in CoL , B. geelongensis View in CoL , B. mocamedesensis , B. sinusaquilensis View in CoL .
(2) Dorsally arched, semicircular to subtriangular, with continuously rounded outlines, not caudate: B. sp. 2 (herein), B. angolensis View in CoL , B. balihaiensis View in CoL .
(3) Caudate to sinuate, with smoothly arched to sinuous dorsal margin: B. sp. 2 (herein), B. sp. 3 (herein), B. sp. 4 (herein), B. alcyonicola View in CoL , B. cratericola View in CoL , B. cushmani View in CoL .
These shape tendencies help to distinguish species of Bairdoppilata View in CoL from some other genera of Bairdiidae View in CoL : Neonesidea View in CoL (teardrop-shaped, with more symmetrical LV and RV), Aponesidea View in CoL (flatiron-shaped, with flat venter), Havanardia View in CoL (angulate, flat venter with ventrolateral keel), Mydionobairdia View in CoL (box-shaped, tuberculate); Triebelina View in CoL (rhomboidal box-shaped, punctate, ridged). Confusion arises because similar carapace outlines are found in some species of Paranesidea View in CoL , which can be distinguished from Bairdoppilata View in CoL only by close attention to details of hingement, patch pattern, surface ornament, appendages and genitalia.
Hinge. The functional hinge is a straight median bar where the thickened edge of the RV fits into a groove beneath a ledge in the LV. The anterior and posterior ends of the bar are expanded ventrally and may be slightly elevated. The corresponding terminal sockets of the LV are shelf-like, without a ventral confining ridge.
In calcified valves of some species, the dorsal surfaces of both the bar and the groove may be finely to coarsely striate. This serrate texture was first illustrated by Morkhoven (1958, Pl. 46, figs. 4–6) for G. co ro n a t a, where it is dramatically expressed. In that thick-walled species the “exceedingly minute striations under favorable lighting” ( Stephenson 1946, p. 346) are vertical, regular, and sharply incised. The band of striations thins at both ends to wrap dorsally over the LV sockets, where it is reflected as complementary indentations across the terminal hinge teeth of the RV ( Morkhoven 1958, Pl. 46, figs. 5, 6). A somewhat similar effect occurs in Macrocyprididae View in CoL ( Triebel 1960, Pl. 14, figs. 4–10; Maddocks 1990, Pl. 60, figs. 1–9, Pl. 61, figs. 1–9). Crenulate hinge texture in Bairdiidae View in CoL was illustrated for Neonesidea schulzi ( Hartmann, 1964) View in CoL , Neonesidea michaelseni Hartmann, 1984 View in CoL , and Bairdoppilata mocamedesensis by Hartmann (1964, Pl. 5, figs. 20, 21; 1974, Pl. 20, figs. 150a, b; 1984, Pl. I, fig. 9). Titterton & Whatley illustrated striate hinges for four more species of Neonesidea View in CoL and Bairdoppilata View in CoL (1988, Pl. 1, figs. 8, 15; Pl. 2, figs. 6, 16).
This striate zone is a microstructure within the carapace wall, rather than an articulation surface. It marks the uncalcified connective zone along the midline where only the chitin ligament connects the valves (Maddocks 1990, 1995). The term ligament was re-established for Ostracoda by Kornicker (1969). Its distinct ultrastructure as an independent element of the carapace was demonstrated by Yamada (2007b), who sectioned the hinges of Neonesidea oligodentata (Kajiyama) View in CoL and Triebelina View in CoL sp. On this basis, he classified the hinge of Bairdioidea View in CoL as “exterior type,” because the overlap structure (edge of LV) develops dorsal to the ligament.
In the decalcified carapaces studied here, the contact zone between the valves displays as two narrow cords or ribbons of chitin (Fig. 9G–J, 17P, 21K). This connective band is strong. The dissected specimens ripped through the fabric of the adjacent valve wall rather than separating along the midline ( Figs. 12 View FIGURE 12 E–F; 16A; 20A, L). In some Bairdiidae View in CoL these cords appear to be straight ( Paranesidea View in CoL sp. 2, Fig. 21 View FIGURE 21. P K–L; unpublished images of Neonesidea tenera View in CoL ). In B. scaura View in CoL , B. sp. 2 and B. sp. 4 each ribbon is sinusoidally rippled from anterior to posterior (Figs. 9I –J, 12A, 17P). It is likely that this rippled band of chitin confers the striate texture to the hinge in a calcified specimen. Hartmann published an unusual SEM view of the hinge region in a well calcified valve of B. sinusaquilensis View in CoL (1979, Pl. I, figs. 16–18). It shows a row of tiny globular projections within the calcified fabric of the hinge zone, which may be the lateral meander-edges of these zig-zag ribbons.
At the anterior and posterior ends of the hinge zone in dorsal view in B. scaura View in CoL , this chitinous band expands across the midline into the LV and becomes more coarsely scalloped (Figs. 9G, I–J). These scallops are interpreted as tiny, crenulate teeth, just beyond and dorsal to the terminal shelf-sockets of the LV hinge. A similar, crenulate, dorsal end-tooth is visible in B. sp. 2 ( Fig. 12 View FIGURE 12 A, at the far right of the image, beyond the anterior socket-shelf). In B. sp. 4 these terminal teeth are evident but more subtle ( Fig. 17 View FIGURE 17. A – F O). A five-part LV hinge with unusually deep, loculate terminal sockets and tiny, crenulate terminal teeth, as well as a serrate median element, was illustrated for Neonesidea michaelseni View in CoL by Hartmann (1984, Pl. 1, figs. 6–11). The differentiation of terminal hinge teeth and sockets is a common device in Ostracoda to minimize valve offset, and one may speculate that Bairdiidae View in CoL living in high-energy environments would benefit thereby.
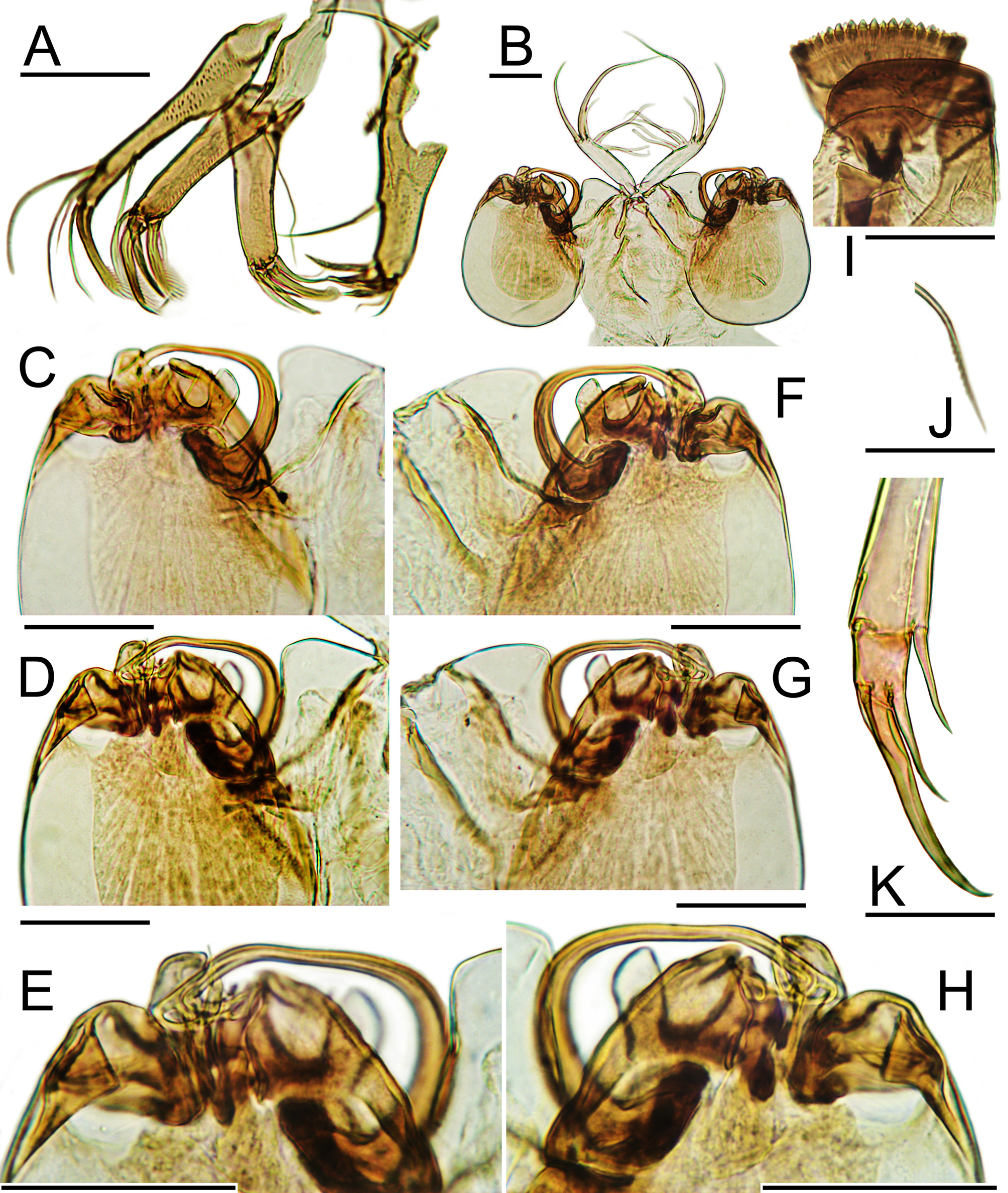
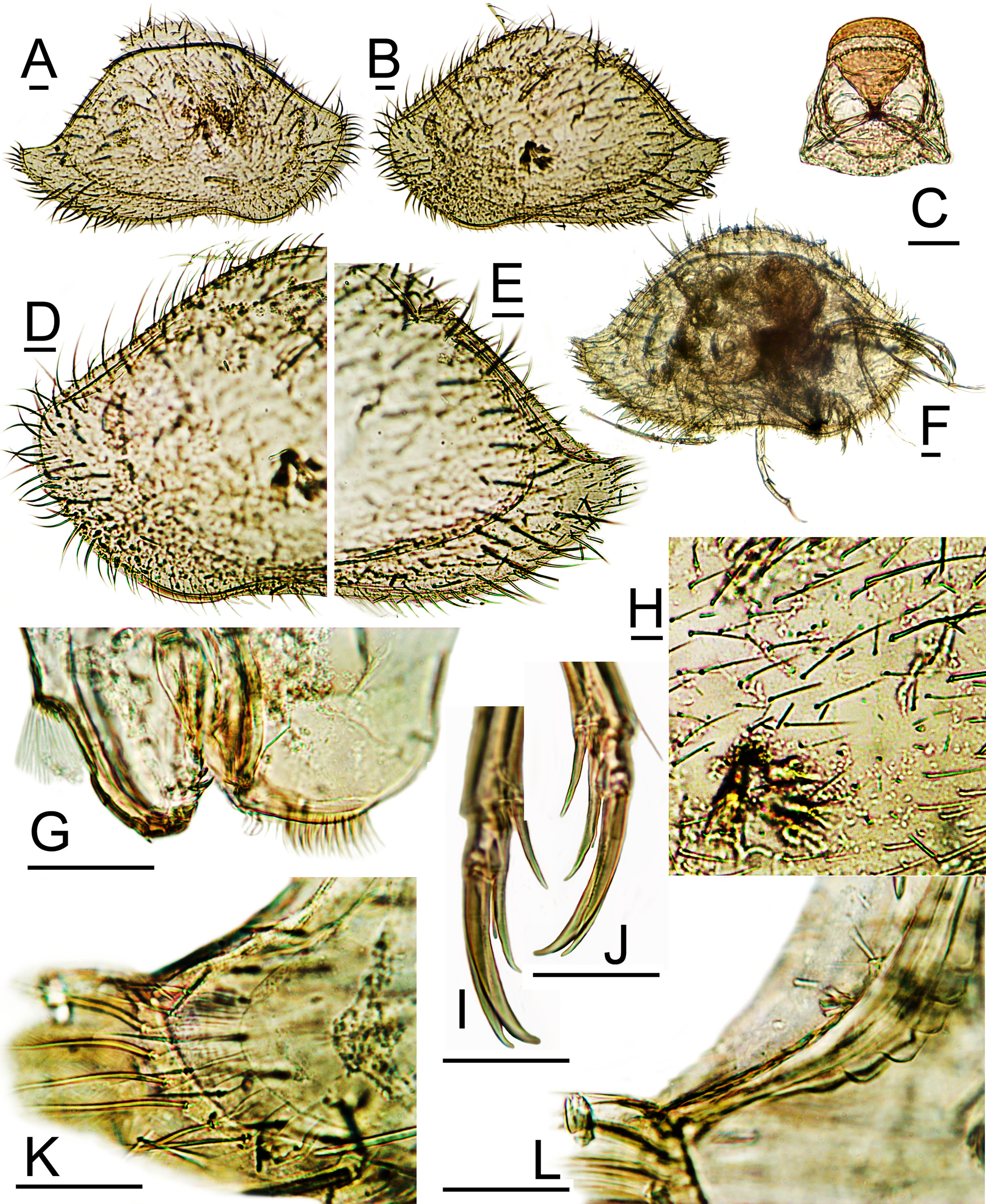
Accessory bairdoppilatan dentition. In calcified specimens, accessory bairdoppilatan dentition is clearly seen in both valves. On the anterodorsal and posterodorsal marginal infold (duplicature) of the LV, beneath the dorsal overhang, the surface swells into a small crescentic platform, in which are four to six depressions. Small teeth project from the anterodorsal and posterodorsal edges of the RV and fit into these depressions when the valves are closed. The posterior platform of the LV is located at (beneath) the slight concavity in the posterodorsal silhouette, where the posterodorsal margin meets the caudal process. The anterior platform is located at (beneath) the corresponding point in the anterodorsal silhouette, although most species of Bairdoppilata View in CoL have a continuous anterodorsal slope rather than a concavity. In the decalcified specimens studied here, the chitinous fabric retains sharp outlines of the teeth and sockets ( Figs. 8 View FIGURE 8 E–F, 9H, 12E–F, L, 16L, 17A).
Normal pore canals. Smith & Kamiya (2002, Fig. 5 View FIGURE 5 ) documented the ontogenetic increase of normal pore canals for the LV of Neonesidea oligodentata View in CoL , from 10 pores in the A-7 instar to 1485 in the A-1 instar and 2145 pores in the adult. Over the central regions of the valve, newly added pores are sprinkled between the pores inherited from the previous instar, but along the free margin pores are crowded together densely, nearly touching in the adult.
Simple normal pores of many sizes are abundant in Bairdoppilata View in CoL . The larger pores, which probably arose earlier in ontogeny, are fewer in number and have distinct muri (9C, F, 17P). Pores of smaller diameter have narrower muri or none (8D, 16H). Simple (rimless), tunnel-shaped (with narrow rims) and funnel-shaped (with broad sloping rims) NPC were reported in Bairdiidae View in CoL by Puri & Dickau (1969, types A’ and A), Puri (1974), and Keyser (1980).
Radial pore canals. The radial pore canals of Bairdoppilata View in CoL are exceptionally numerous, remarkably straight, and very closely spaced ( Figs. 8 View FIGURE 8 G–I, 9E–F, 16K). Each canal originates in the vestibule (lined with epidermis) and ends in a pore with an associated seta. Some lead to marginal pores and setae, but many are so-called false radial pore canals, leading to pores and setae clustered near but not quite at the valve edge ( Figs. 8 View FIGURE 8 H–I). There are no differences in thickness of the canals, and the distinction between true and false radial pore canals is probably not significant. Perfectly straight RPC are also evident in Paranesidea View in CoL sp. 1 ( Fig. 20 View FIGURE 20 C, L–M, S).
Exterior carapace setae. Numerous simple setae (sensilla) of many sizes have been observed in these species of Bairdoppilata View in CoL ( Figs. 8 View FIGURE 8 A, D, 12H; 13B, F, 16A–B; D–E, H), but no branching (polyfurcate) setae, no barbed or thorny setae, and no anchor setae ( Maddocks 2013). All have limited flexibility and taper to a sharp point. Most are oriented more or less posteriorly. The color is light to medium brown in these specimens. Because the setae are less conspicuous than in some species of Neonesidea View in CoL , although their numbers and density are comparable, shallowwater species of Bairdoppilata View in CoL are less likely to have been described as "hirsute."
Marginal carapace setae. Three kinds of marginal setae (sensilla) have been observed in the species of Bairdoppilata View in CoL studied here: simple setae, eyelash setae and plumose setae. No caudal setae have been seen. A fifth kind (pappose setae) has not been seen in Bairdoppilata View in CoL but is present in a species of Paranesidea View in CoL . These terms, of which two were defined by Broodbakker & Danielopol (1982, in context of appendage chaetotaxy) and three were proposed by Maddocks (2013), are used here in the descriptive sense, with no implications regarding homology. Because of the large number and crowded spacing of pores and setae in the marginal zone, individuals are difficult to recognize, and it is not known whether an individual seta may change its morphologic expression during ontogeny.
Simple setae are setae on the carapace exterior (so-called outer lamella), some of which happen to be located near the valve edge ( Figs. 8 View FIGURE 8 H–I, 9C–F, 16D–E, K). They include some of the ontogenetically oldest (hence longest, thickest and darkest) carapace setae. They originate from false radial pore canals at pores with larger diameters and distinct, circular muri, near the edge of the valve on the exterior surface. They are stiff, smooth, nearly straight and taper to a point. Their diameter is greater than eyelash setae or plumose setae, and they are longer and darker in color.
Eyelash setae are short, thin, straight or weakly curved setae, which are aligned at regular intervals in a single row and parallel, like pickets of a fence (Figs. 9C–D, F; 16D–E). They are light-colored and taper to a point. They originate from pores at the edge of the calcified zone, along the base of the selvage-ridge, but inside the chitinous flange, if one is present (Figs. 9C–D, 12E). They are about half as long (or less) as any nearby plumose setae. Their form is the same as simple setae, with a basal constriction and ring-like collar. Although many pores are crowded close together in this marginal zone, the pores belonging to eyelash setae may be recognized by their location closest to the edge and their regular spacing. They are thought to open on the interior surface, although that fact has little significance for homology, because the marginal infold is a continuous fabric ( Harding 1964, Kornicker 1969, Yamada 2007a). An early illustration showing relationships of simple setae, eyelash setae and radial pore canals is that of Müller (1894, Pl. 15, fig. 9, for a species of Neonesidea View in CoL ). Hartmann (1974, Pl. 17, fig. 127) illustrated these two sizes of marginal setae for B. angolensis View in CoL . For additional illustrations see Maddocks (2013, Figs. 8 View FIGURE 8 G–H, 18H, 24G–H, 25F, 29C, 30C–E).
Plumose setae are short, thin setae, edged by two rows of fine, regularly spaced, parallel barbs or vanes that give them a plume-like or feathery appearance (Figs. 9F, 12F, K, 16K, 17L–M). They are light-colored and somewhat flexible. Where present, they are spaced at regular intervals and have consistent lengths (twice as long as nearby eyelash setae, or longer). They open from regularly spaced pores near the edge of the calcified zone on the inner surface, along the outward-facing edge of the selvage-ridge. The barbs are fine, barely detectable microscopically, nearly straight, uniformly parallel, and oriented at about 45o to the shaft. Some suspected plumose setae are thought to have barbs that are too small to be seen; these plumose setae are discernable by their beaded aspect, gentle curvature, relative length and tendency to attract detritus ( Figs. 12 View FIGURE 12 E, 17A).
The term plumose seta was defined by Broodbakker & Danielopol (1982, p. 106, Fig. 2 View FIGURE 2 C) as a division of plumed seta in which “the setules are hairy and have a flexible appearance.” Their analysis of homologies was documented by SEM photos of appendage setae. Published drawings of plumose setae as marginal carapace setae of Bairdiidae View in CoL include Müller (1894, Pl. 15, figs. 8, 10) and Maddocks (2013, Figs. 5 View FIGURE 5 E–F).
Plumose setae were documented for two species in the Neonesidea pateriformis View in CoL species-group by Maddocks (2013), N. tenera Brady (1886) View in CoL and N. plumulosa Maddocks (2013) View in CoL . In that publication they were labeled as “caudal setae” (perhaps a less-developed form), but it now seems appropriate to distinguish them by a more precise term to avoid speculation concerning homology. In those two species, both simple setae and plumose setae are located in close proximity at the caudal region, on the exterior and interior surfaces, respectively, but there are no caudal setae present ( Maddocks 1995, Figs. 5 View FIGURE 5 A–D; 8D–E; 19A–B, D).
In the four species of Bairdoppilata View in CoL studied here, about eight plumose setae are visible at the top of the posteroventral free margin, in the caudal region of both RV and LV. In B. sp. 2, additional plumose setae are suspected to occur on the anterior margin ( Fig. 12 View FIGURE 12 E). Where eyelash setae and plumose setae occur together along the same margin, the plumose setae are thicker, longer, arise a little farther from the margin, and probably originated earlier in ontogeny.
Caudal setae are large, fan-shaped carapace setae, which open from wide but rimless normal pores on the dorsal exterior surface of the caudal process ( Maddocks 2013). The adjacent valve surface may be slightly swollen to form what is called a “humped caudal process.” They have unusually thick basal shafts and broad, flat, terminal fans with fringed edges. They are well developed in the Neonesidea schulzi View in CoL species-group, just dorsal to the posterior spine. Caudal setae were documented for one Hawaiian species ( Neonesidea holdeni Maddocks 2013 View in CoL , Figs. 25F–I, 26H).
Caudal setae have not been observed in Bairdoppilata View in CoL . In the species studied here, the simple setae on the exterior dorsal surface of the caudal process have no visible barbs. The normal pores in this location are not enlarged, and the surrounding surface is not swollen. The LV with caudal setae illustrated for B. portsamsonensis View in CoL by Hartmann (1978, Pl. 1, fig. 14) belongs to a species of Neonesidea View in CoL .
Pappose setae were defined by Broodbakker & Danielopol (1982, p. 106) for plumed setae in which the setules arise on all sides of the shaft. On carapaces of Bairdiidae View in CoL , they were first illustrated by Kornicker (1961, Text-figs. 10A, D) for Paranesidea gigacantha View in CoL . They are not restricted to the caudal region but occur around the free margin. In Paranesidea View in CoL sp. 2, the suspected pappose setae are thin, short (about 30 µm), originate at pores on the outer face of the selvage-ridge, and reach across the chitinous flange but not beyond it ( Figs. 21 View FIGURE 21. P M–Q). They are not visible from the exterior. The shaft is thicker at the base and tapers. The barbs or setules appear to arise all around the shaft, rather than in just two lateral rows, giving it a tree-like or root-like rather than feather-like appearance. None have been observed in Bairdoppilata View in CoL .
In Fig. 21 View FIGURE 21. P M for Paranesidea View in CoL sp. 2, about 9 pappose setae emerge from regularly spaced, arched, tunnel-like pores at the crest of the selvage-ridge, along the anterior margin. Several more pappose setae (out of focus) emerge at lower elevations on the selvage-ridge. The six curved setae at the base of this image are eyelash setae, which emerge at the base of the selvage-ridge and extend beyond the chitinous flange. A separate nerve canal (RPC) leads to each seta. In total, this image includes at least 15 pappose setae plus 6 eyelash setae. Figs. 21 View FIGURE 21. P N–Q are enlargements of this same region, at different levels of focus, showing details of these moss-like pappose setae.
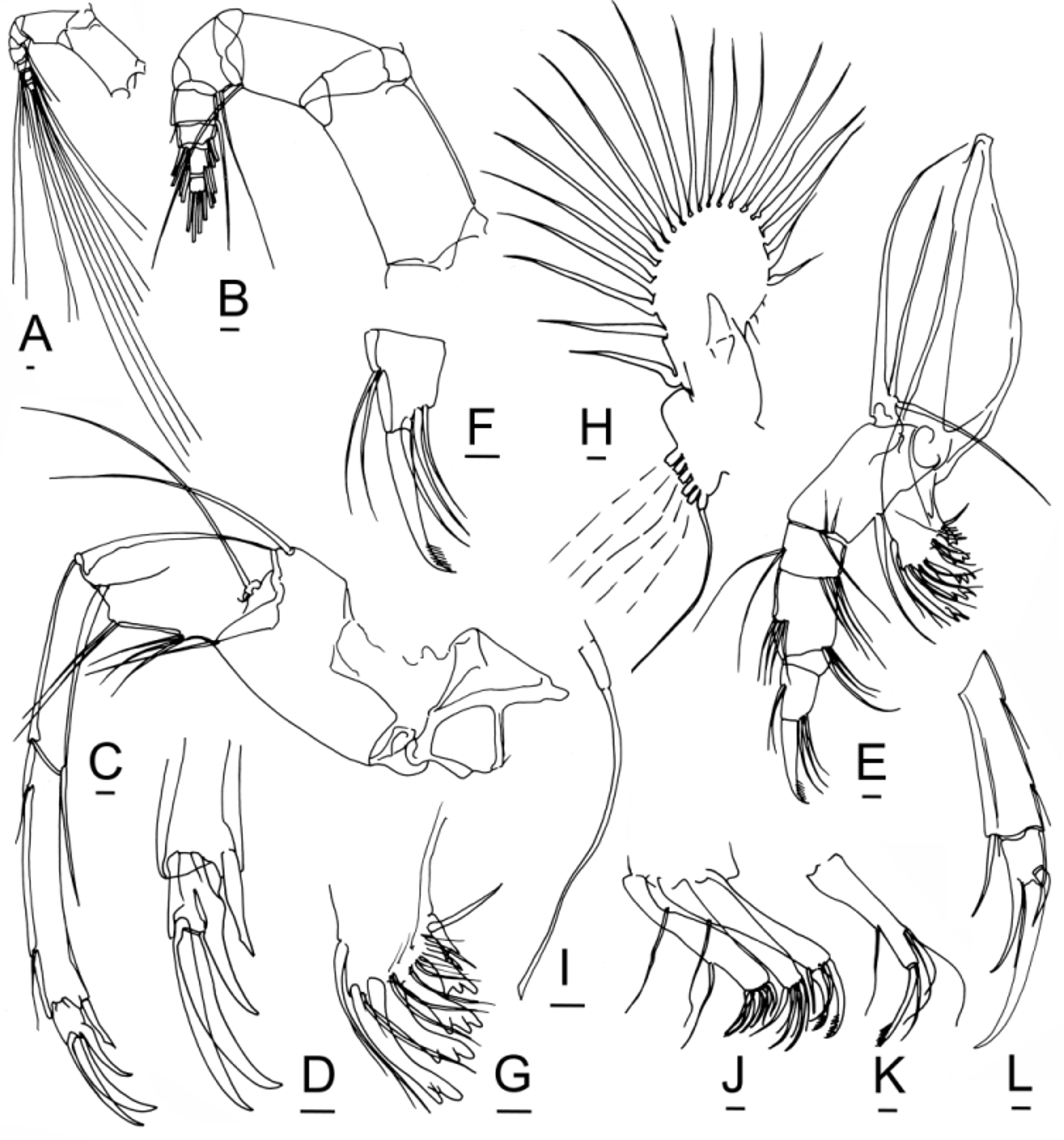
Antennule. The antennules of Bairdoppilata View in CoL are constructed according to the normal pattern for the family ( Figs. 6 View FIGURE 6 A–B). As usual, the three distal podomeres are severely reduced and carry a tassel of about 13 very long, flexible, tactile setae. So far as known, they do not provide information useful for discriminating species and genera.
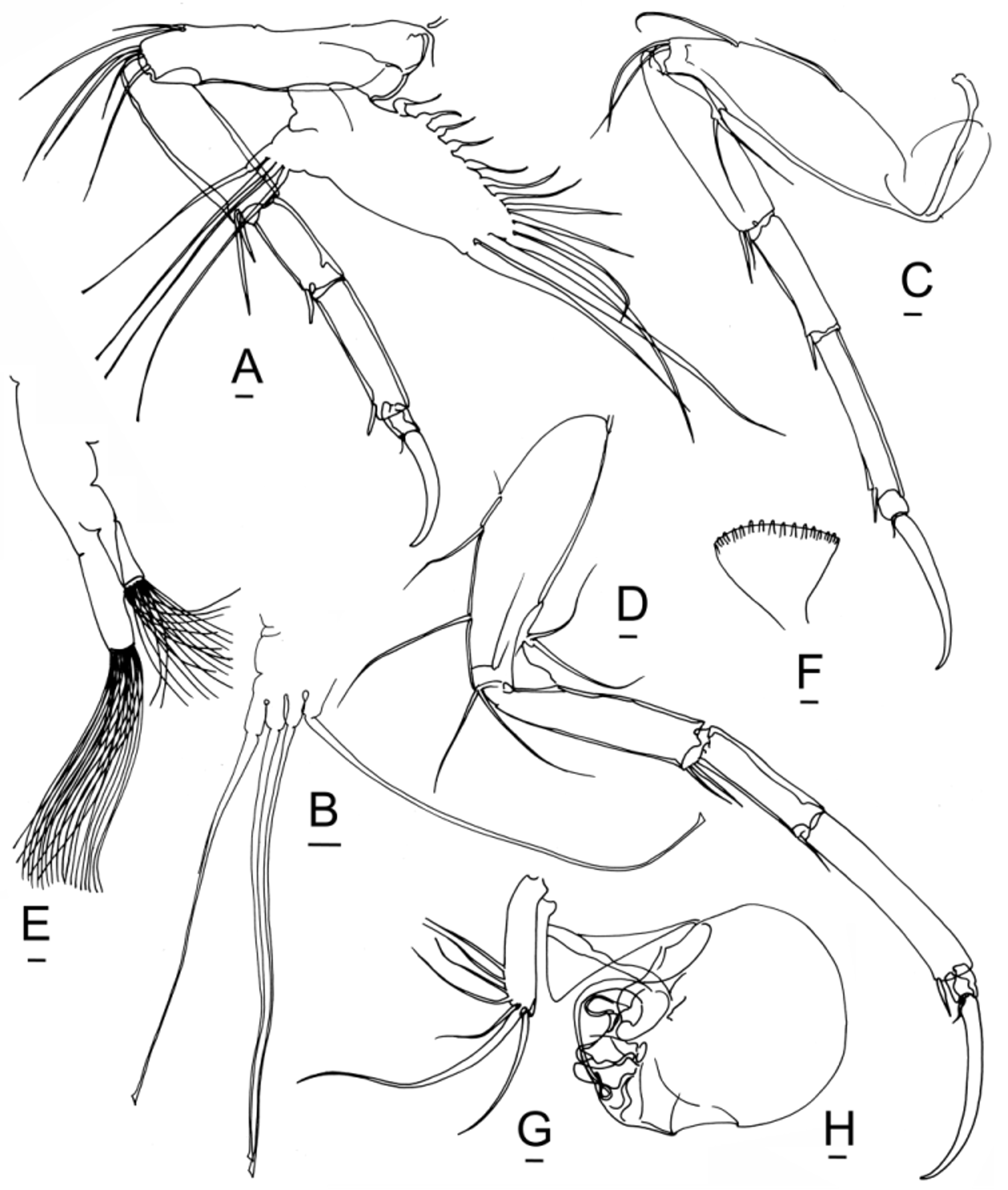
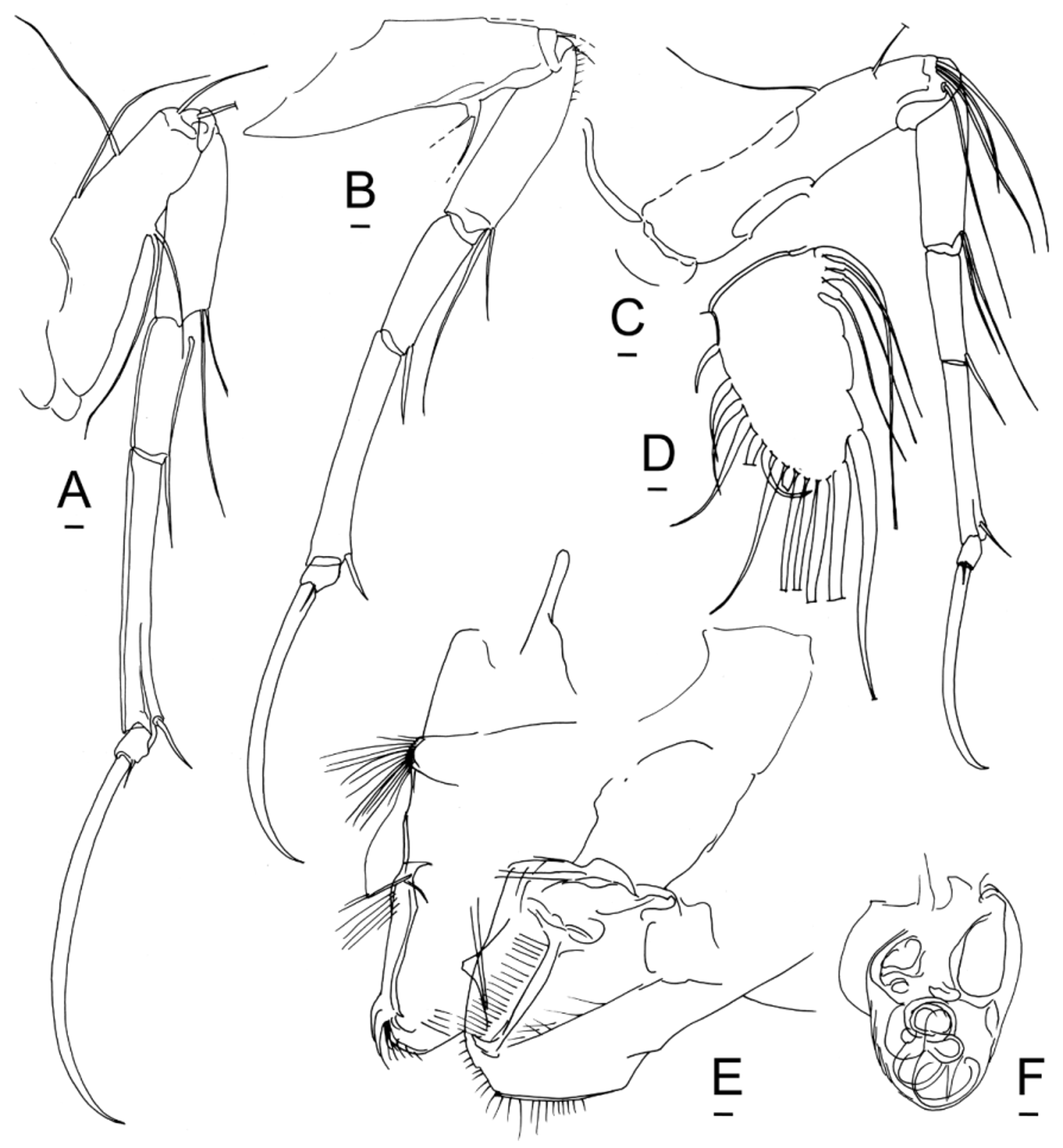
Antenna. The antennae (second cephalic limbs) of Bairdiidae View in CoL are the anterior walking legs, assisted by the three thoracic legs. The sharply pointed, curved distal claws provide a secure toe-hold on steep surfaces, like the crampons of a climber on a glacier. In females of Neonesidea View in CoL , the tip of the main claw is obliquely beveled with a sharp point, and this feature is repeated in the distal claws of the thoracic legs. This beveled configuration resembles the ice tool (technical axe) used by mountaineers to climb a vertical wall of ice. In Aponesidea View in CoL and Havanardia View in CoL the claw tip is less abruptly bent. In Triebelina View in CoL the tips of both the antennal claw and the thoracic legs are barbed. In Paranesidea View in CoL and Mydionobairdia View in CoL the pointed tip merely follows the natural curvature of the claw, like a scimitar. In Bairdoppilata View in CoL and Glyptobairdia View in CoL the distal claws are relatively broad and bladelike, curving smoothly to rounded or pointed tips.
The antenna of Bairdoppilata View in CoL and Glyptobairdia View in CoL has two terminal claws of the same or nearly the same size, resembling a pair of scissors ( Fig. 6 View FIGURE 6 C–D, 10G, 13G, 14H–I, 16I –J, 17G). In these shallow-water species, the anterior accessory claw has the same curvature and almost the same length and thickness as the main distal claw. In three species of? Bairdoppilata View in CoL described by Brandão (2008) from the Southern Ocean (? B. labiata View in CoL ,?B. sp. 1 aff.? B. labiata View in CoL , and? B. sp. 2 aff.? B. labiata View in CoL ), the anterior accessory claw is noticeably shorter and thinner than the main distal claw. By contrast, in Paranesidea View in CoL and other genera ( Neonesidea View in CoL , Aponesidea View in CoL , Triebelina View in CoL , Havanardia View in CoL and Mydionobairdia View in CoL ) the homologue of this anterior claw is a thin accessory seta ( Fig. 18 View FIGURE 18 A–B; 20J, Q–R; 21E–F). This seta is flexible and sexually dimorphic, longer in females than in males. It is occasionally damaged or overlooked in published drawings.
The accessory claw does not assume this form until the final molt, being represented only by a flame-shaped anlage in the A-1 (pre-adult) instar ( Figs. 6 View FIGURE 6 L, 11K, 13I –J; see also Maddocks 1973, p. 63, Fig. 6 View FIGURE 6 B). In spite of its late emergence in ontogeny, at the same time as many sexually dimorphic traits, it has the same configuration in adult males and females. None of the distal antennal claws are dimorphic in Bairdoppilata View in CoL , although they are in some other bairdiid genera.
Although the accessory claw of Bairdoppilata View in CoL is usually described as enlarged, relative to its development in other living genera of Bairdiidae View in CoL , this might be a primitive or relict condition rather than derived. Bairdoppilata View in CoL is well represented in Cretaceous and younger strata by fossil species (identified by accessory locking dentition), and possible relatives are known in the Triassic.
The bairdiid antenna has 6 podomeres, of which I and II belong to the protopodite and III–VI belong to the endopodite. Podomere I is often omitted or unclear in illustrations, because of damage during dissection, or because its curvature makes it unstable in lateral profile. Between podomeres I and II, a stove-pipe joint allows podomere II to rotate up and in, until the dorsal (outer) edge becomes parallel to the inclined surface of the forehead, near the concave upper portion of the S-shaped strut of the head capsule, a little below the base of the antennule ( Fig. 13 View FIGURE 13 C). A second stove-pipe joint between podomeres II and III provides additional lift and accommodation around the forehead. The exopodite scale, carrying one long and two tiny setae, is tightly attached to the outer proximal edge of podomere III and remains with it if the protopodite is torn away.
At the “elbow joint” between podomeres III and IV, the rigid distal part of the limb can rotate through approximately 74o. It can be raised as high as 126o, and it rotates down past vertical to an orientation of 200o. The limit to which the limb can be raised is set by the anterodorsal marginal overlap of the LV ( Fig. 16 View FIGURE 16 F). At maximum extension in preserved animals, the distal claws, all of podomere VI and about half of podomere V may be exposed outside of the carapace. When the antenna withdraws into the domicilium, the distal claws are held just below the upper lip, parallel to and just within the anteroventral margin of the closed carapace ( Fig. 13 View FIGURE 13 C). The rather straight course of the anteroventral margin in the RV may indicate the orientation of the antennal claws within.
Podomeres IV and V are laterally compressed and aligned in a stiff arc that resists distortion. There is no flexibility at the joint between them, which is set at an oblique angle to the trend of the limb and braced by a clawlike ventrodistal seta. A strong tendon within podomere V confers additional rigidity. The joint between podomeres V and VI has very little movement, because the robust ventrodistal claw of podomere V fits against a basal notch in podomere VI and acts as a stop. The amalgamation of the posterodistal fused claw into podomere VI provides a brace to limit movement of the terminal claws. In this way, the entire distal limb (podomeres IV, V, VI and terminal claws) behaves as a rigid grappling unit, which is operated in lever fashion by the muscles in podomere III (somewhat like a backhoe).
Podomere IV is always shorter than podomere V, but the proportional relationship between them is inconsistent, ranging from 0.58 in G. coronata View in CoL to nearly equal (0.97) in B. hirsuta View in CoL . This is unexpected, because these two podomeres are immovably sutured and function as a unit. This relationship should be investigated in other genera of Bairdiidae View in CoL .
The antenna is the most frequently illustrated limb and is thought to present diagnostic details for the identification of species. Yet, it is surprisingly difficult to extract consistent chaetotaxial information from published drawings because of missing information and probable errors. For a few species, only the most distal podomeres and claws have been figured. For six species, the appendage drawings were published without magnification scales, and their dimensions cannot be compared ( B. angolensis View in CoL , B. balihaiensis View in CoL , B. cytheraeformis View in CoL , B. geelongensis View in CoL , B. mocamedesensis , B. sinusaquilensis View in CoL ). For some species, the illustrated legs belong to a different species than the illustrated valves ( B. portsamsonensis View in CoL , B. simuvillosa View in CoL ). Verbal assessments of shape (long, thin) have limited usefulness, because they lack a context for comparison.
Within these limitations, the antennal chaetotaxy (presence/absence of setae) of Bairdoppilata View in CoL appears to follow the standard pattern for the family: podomere I: 2 long posterodistal setae; podomere II: 1 long anterodistal and 1 shorter posterodistal setae; podomere III: 3 medium posteromedial aesthetascs and 2 posterodistal setae (both long, or one long and one medium); podomere IV: 1 or 2 posterodistal setae (1 long claw, or 1 long claw and 1 short seta); podomere V: 2 or 3 anterior filaments and 1 short posterodistal claw; podomere VI: 1 long anterodistal (accessory) claw, 1 long terminal (main) claw, 1 short terminal aesthetasc, and 1 short posterodistal (fused) claw.
Beyond presence/absence, the relative proportions of these structures may carry taxonomic information. Lengths and widths of antennal podomeres, setae and claws were measured and compiled for the illustrated species of Bairdoppilata View in CoL . Because of numerous gaps (missing information), the full data set (36 illustrations, 22 species, 21 characters) proved unsuitable for systematic analysis. A subset of these data is presented as Table 3. Associative plots (such as Fig. 5 View FIGURE 5 ) suggest that the following dimensions tend to vary together:
(1) Length of accessory claw compared to length of main claw. In the shallow-water species, they are very nearly the same length (always within 10 percent, usually within measurement error).
(2) Length of distal claw of podomere V compared to length of fused claw of podomere VI, and (3) Length of distal claw of podomere IV compared to length of podomere V, and (4) Length of distal claw of podomere V compared to length of podomere VI. Each of these claws acts as a brace to prevent excessive movement of the following podomere. The consistent size relationships support this functional hypothesis.
(5) All widths of distal podomeres and widths of claws.
These results suggest that drawings showing only the distal claws (like Figs. 6 View FIGURE 6 D and 14I) do not provide as much independent taxonomic information as is commonly supposed. More proximal setae and podomeres might be more distinctive, but they are also more frequently damaged or omitted from illustrations.
Explanation of abbreviations: NO = number, NAME = species name or informal identification, SPEC = catalog number of specimen, SEX = sex of illustrated specimen, M = male, F = female, REF = published reference, FIG = figure number of measured illustration, LOC = locality, LCAR = carapace length (µm), HCAR = carapace height (µm), H:L = Height:Length Ratio of carapace, DEPTH = water depth in meters, MAIN = length of main (terminal) claw, ACC = length of anterior accessory claw, FUS = length of fused claw of podomere 6, P5C = length of posterodistal claw of podomere 5.
sp1 aff lab =? Bairdoppilata View in CoL sp. 1 aff.? B. labiata View in CoL ; sp2 aff lab =?B. sp. 2 aff? B. labiata View in CoL ; sp. 2 = B. sp. 2 (this paper), sp. 3 = B. sp. 3 (this paper).
B2008 = Brandão 2008, M1969 = Maddocks 1969, M1973 = Maddocks 1973, M1975 = Maddocks 1975, R1960 = Rome 1960, Herein = this paper.
E25 = Eltanin 25, 0 4o 53’N, 80o28’W to 0 4o 51’N, 80o28’W, east of Galapagos Islands; E418 = Eltanin 418, 62o39–40’S, 56o8–10’W, Antarctic Peninsula; E1345 = Eltanin 1345, 54o50–51’S, 129o46–48’W, Pacific Sector of Southern Ocean; E1418 = Eltanin 1418, 54o32’S, 159o02’E, Kerguelen Island; FFS = French Frigate Shoals, the Hawaiian Islands; FL = Florida Keys; GAUS = Gauss-Station, Antarctica; GM = Gulf of Mexico; NB = Nosy Be, Madagascar; SB = St. Barthelmy Island, Lesser Antilles; BAH = Bahama Islands, ASC = Ascension Island; WED = Weddell Sea, Antarctica.
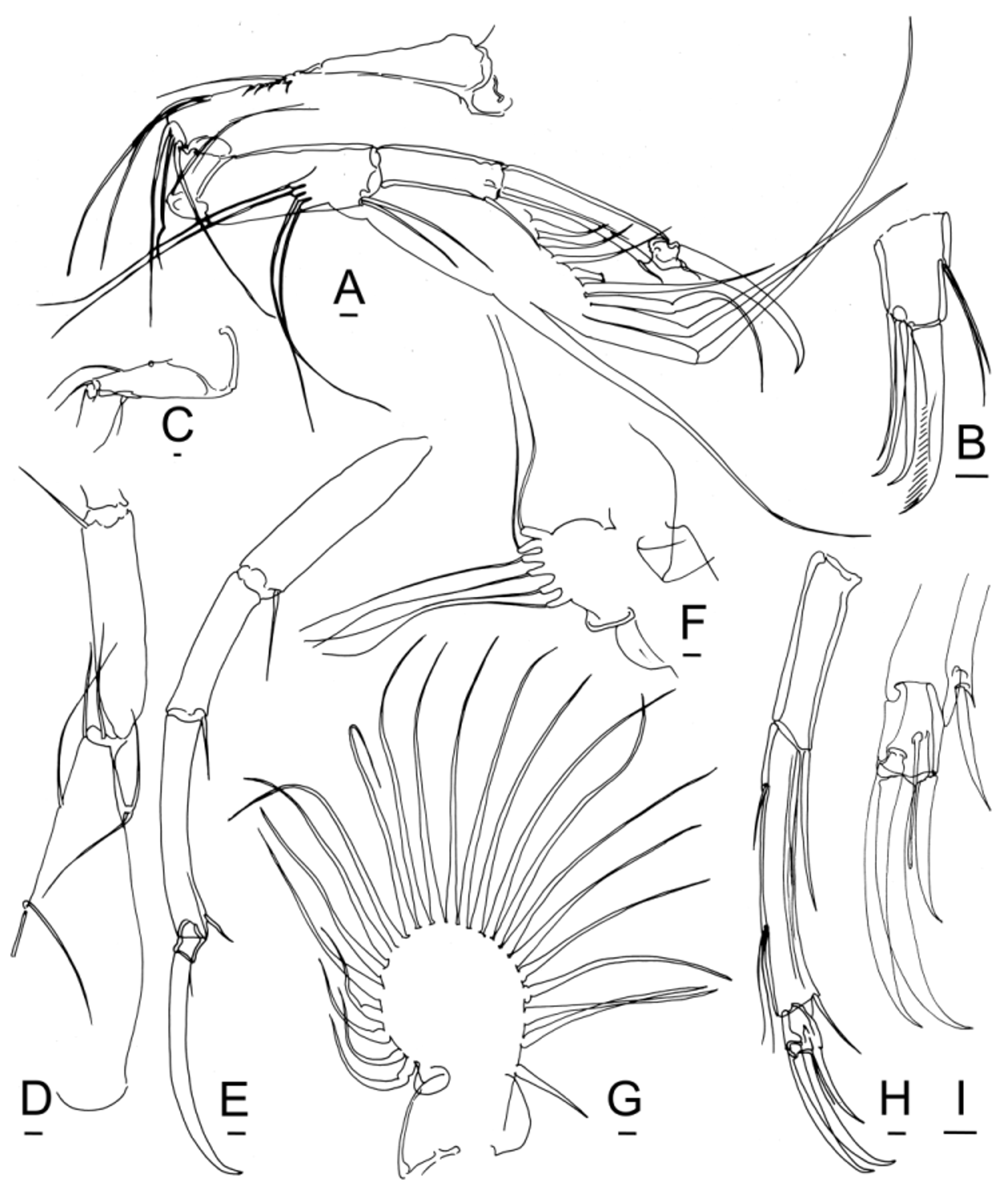
Mandibles. So far as known, the mandibles of Bairdoppilata View in CoL and Paranesidea View in CoL are consistent with the familial pattern ( Figs. 6 View FIGURE 6 E–G, 14B, 15B, 18D), although this limb has not been illustrated for many species. The terminal claw of the palp is distally fringed or pectinate, which offers a potential site for taxonomic or diet-related variation ( Fig. 6 View FIGURE 6 F, 14B, 21B). The palp is thought to move food into the mouth. The basal teeth are inserted into the atrium from the sides and chop or partition the food into bites.
Maxillae. The palp of the maxilla (maxillule) and the three masticatory processes (endites) are held nearly horizontal along the boat-shaped ventral surface of the lower lip ( Figs. 13 View FIGURE 13 C, F) and may act as a brush to sweep food toward the atrium. The outer integument may have a prickly texture with rows of papillae or tiny setules ( Figs. 10 View FIGURE 10 H–I, 11A). As far as known, the chaetotaxial pattern described below for Bairdoppilata View in CoL is the same as in Paranesidea View in CoL and other genera of Bairdiidae View in CoL . There is inconsistency in published illustrations, especially in smaller species, either because the number of setae is reduced or because of difficulties of observation. Details of this configuration might have taxonomic significance, but there is insufficient documentation at present.
The slender, unjointed palp carries a cluster of three setae at the anteromedial indentation, a posteromedial seta, a terminal seta, and a stout, fringed or pectinate terminal claw (6K, 10I, 15A, 18E, 20H, 21B). The first masticatory process carries a posteromedial seta and 6 or more terminal setae, of which one is a bristle (6J, 10H, 15A, 20G, 21C). The second masticatory process has a posteroproximal seta, a fringed or pectinate terminal claw, which may be spatulate or spoon-shaped, another claw, a bristle, and 3 or more other setae (6J, 10H, 15A, 20F, 21D). The third masticatory process has a posteroproximal seta, two fringed or pectinate terminal claws, which may be spatulate or spoon-shaped, another claw, and up to three additional setae (6J, 10H, 15A, 20E, 21D).
The vibratory plate has been illustrated for only two species of Bairdoppilata View in CoL , and two more are added herein. B. alcyonicola View in CoL has 25 plumose setae ( Maddocks 1969, Fig. 38A). Brady (1880, Pl. 5, fig. 2d) drew 22 setae for B. villosa View in CoL . B. scaura View in CoL n. sp. has at least 20 setae ( Fig. 6 View FIGURE 6 H, damaged). B. sp. 3 has 23 setae ( Fig. 14 View FIGURE 14 G). In Neonesidea View in CoL this structure has either 25 or 26 setae ( Maddocks 2013). Smith et al. (2005) suggested a range of 24 to 26 for genera of Bairdiidae View in CoL .
The ventral accessory plate of the maxilla in Bairdoppilata View in CoL carries 6 stiff reflexed setae with wedge-shaped terminations, as usual for this family ( Fig. 6 View FIGURE 6 I).
Thoracic legs. The vibratory plate of the first thoracic leg in Bairdoppilata View in CoL is thought to have 13 plumose seta, as in Neonesidea View in CoL and other genera of Bairdiidae View in CoL ( Fig. 7 View FIGURE 7 A, Table 4). For four species, only 12 plumose setae have been reported, however, as also is the case in Paranesidea View in CoL sp. 1 ( Fig. 19 View FIGURE 19 D).
The four reflexed setae are segregated anteriorly, well separated from the plumose setae. Hartmann (1978) reported only 3 reflexed setae in B. balihaiensis View in CoL . As far as known, they are similar in males and females, not sexually dimorphic as in Neonesidea View in CoL . In Bairdoppilata View in CoL the first reflexed seta may be a little shorter than the others ( Figs. 7 View FIGURE 7 A–B). In Paranesidea View in CoL sp. 1 the first seta is actually longer than the others ( Fig. 19 View FIGURE 19 D). They are smooth, somewhat stiff and end in oblique, wedge-shaped, flat tips, as described for Neonesidea ( Maddocks 2013) View in CoL .
On the second and third thoracic legs, the corresponding, vestigial epipodite consists of two thin, unequal setae, fused at their base.
Explanation of abbreviations: ALCY = B. alcyonicola View in CoL , BALI = B. balihaiensis View in CoL , CRAT = B. cratericola View in CoL , HIR1 = B. hirsuta View in CoL , USNM 121353, HIR2 = B. hirsuta View in CoL , USNM 139891; MOCA View in CoL = B. mocamedesensis ; SIMP = B. simplex View in CoL ; VILL = B. villosa View in CoL ; SP2A = B. sp. 2, Ascension; SP2L =?B. sp. 2 aff. B. labiata View in CoL ; CORM = G. coronata View in CoL ; CORR = G. co ro n a t a; SCAU = B. scaura View in CoL ; SP 2 = B. sp. 2; SP 3 = B. sp. 3; SP 4 = B. sp. 4.
T5, T6, T7 = first, second or third thoracic leg; I–V = podomeres; anteroproximal (etc.) = location of seta; vib = vibratory plate; plumose = number of plumose setae; reflexed = number of reflexed setae; shorter = whether the first reflexed seta is shorter than others.
B2008 = Brandão 2008, H1974 = Hartmann 1974, H1978 = Hartmann 1978, M1969 = Maddocks 1969, M1973 = Maddocks 1973, M1975 = Maddocks 1975, R1960 = Rome 1960, here = this paper.
The chaetotaxy of the thoracic legs for Bairdoppilata View in CoL follows the standard pattern for the family with no sexual dimorphism ( Table 4; Figs. 7 View FIGURE 7 A, C–D, 14C–E). Claw lengths are relatively shorter and width:length proportions of podomeres are less elongate in the shallow-water species of Bairdoppilata View in CoL than in some other genera. Because of inconsistencies in published illustrations (missing setae and discrepancies in relative lengths of setae), these limbs do not contribute much to identification of species and genera at the present time. Paranesidea View in CoL sp. 1 follows the same chaetotaxial pattern, although both podomeres and setae appear to have more elongate proportions ( Figs. 19 View FIGURE 19 A–D).
The brush-shaped appendage of the male is asymmetrical in Bairdoppilata View in CoL , as usual in the family ( Fig. 7 View FIGURE 7 E).
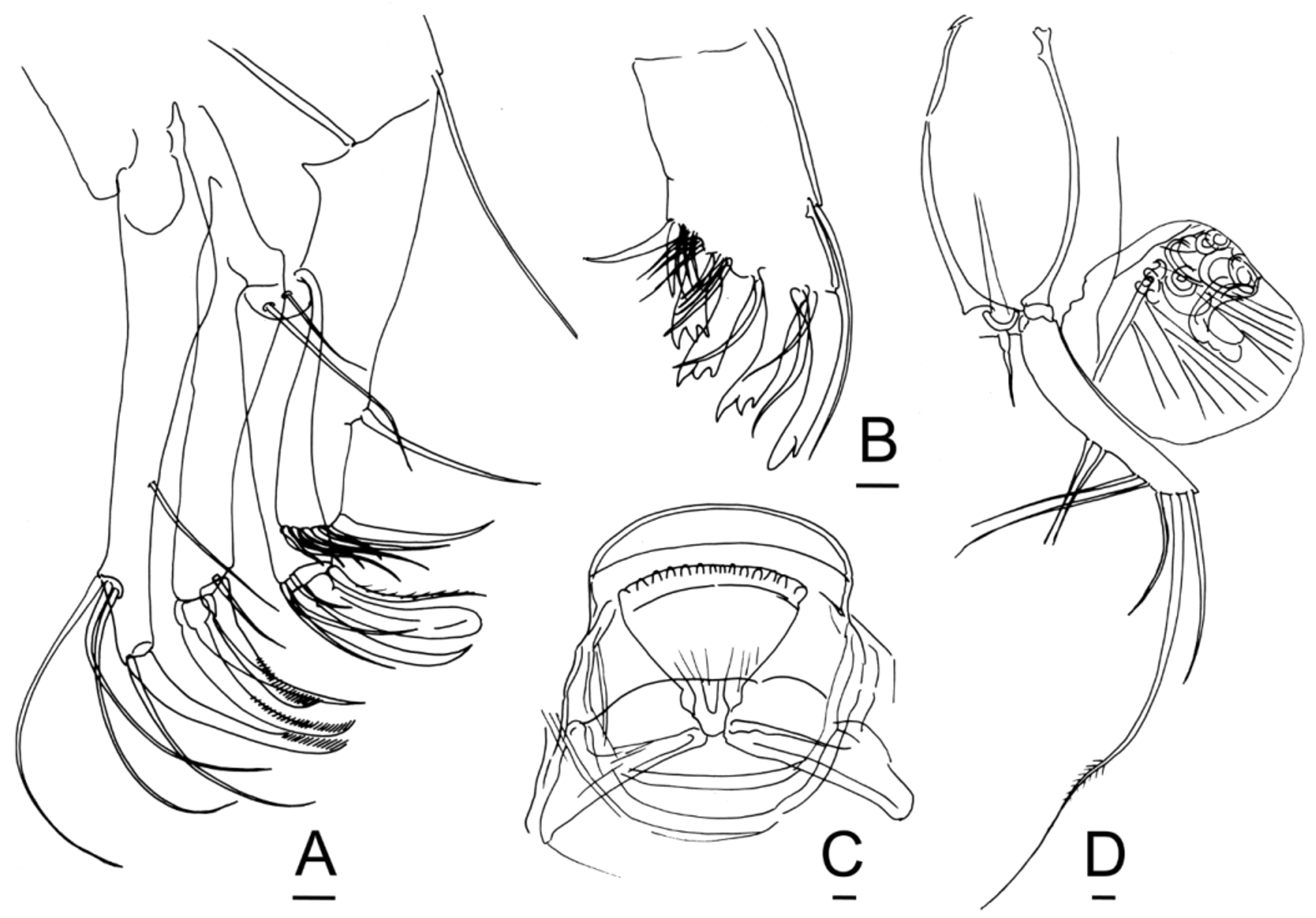
Zygum. The two furcal rods, the single post-abdominal bristle, and the paired external genitalia (hemipenes or genital lobes) of Bairdiidae View in CoL articulate to a median plate or frame, the zygum, which is carried on the midline at the posterior end of the body. This central frame is lozenge- or shield-shaped and heavily muscularized ( Figs. 10 View FIGURE 10 A–B, 11B). It has been illustrated for males many times ( McKenzie & Swain 1967, Text-Fig. 3h; Maddocks 1969, Fig. 6 View FIGURE 6 B, 7D, 9H, 10B, 11A, 12F, 17B, 21E, 24E, 25C, 28F, 30G, 37C, 39H, 41C; Danielopol 1972, Fig. 3 View FIGURE 3 ; Maddocks 1975, Fig. 3 View FIGURE 3 C; Maddocks & Iliffe 1986, Fig. 7 View FIGURE 7 G, 9A, 11A; Maddocks 2013, Figs. 10 View FIGURE 10 A–B, 14E, 19G, 31E, 36M), but its presence in females has rarely been mentioned ( Maddocks 1969, Figs. 31G; 1975, Fig. 7 View FIGURE 7 A). The skeletal frame of the zygum consists of two narrow, flat, arcuate, lateral bars and a short posterior cross-bar. In some specimens the lateral bars may be less well defined along the anterior edges of the plate, or they may be replaced by converging chitin fibers ( Fig. 10 View FIGURE 10 B, 11B). In the specimens examined, there is no skeletal connection betweem the zygum and the lateral framework of chitinous struts, from which the thoracic legs are suspended.
In males the zygum is posterodorsal, and the hemipenes are suspended from it on either side ( Fig. 8 View FIGURE 8 C, 10A–B). This U-shaped apparatus is separate from the posteroventral region of the body and hangs in saddlebag fashion, with the distal ends of the hemipenes pointing posteriorly (Fig. 9A–B, 10A; see also Maddocks 2013, Figs. 10 View FIGURE 10 A–B, 35C, for Neonesidea View in CoL ). The anterior connection to the body is a wrinkled tube ( Maddocks 2013, fig. 20B, for Neonesidea View in CoL ). In use the connecting tube inflates, the whole apparatus extrudes posteriorly (but not the posteroventral part of the body), and the hemipenes unfold through 180o.
In females the zygum is oriented approximately vertically, behind the swollen rump containing the internal female organs ( Figs. 12 View FIGURE 12 G, 13A–B, 16F, 17A, E). The genital lobes arise close to the end of the zygum, but no direct skeletal articulation has been observed ( Figs. 15 View FIGURE 15 D, 17P; see also Maddocks 2013, Figs. 9F–G for Neonesidea View in CoL ). Less musculature is apparent, because in females the zygum is not extrudable.
In Cytheroidea, this rectangular frame between the hemipenes was first illustrated for Hemicytheridae View in CoL by Skogsberg (1928, Pl. 6, fig. 1). The term zygum was designated by Hart & Hart (1969, 1974). In Entocytheridae View in CoL three additional skeletal structures are located anterior to the zygum: a pair of small bars (sterinx), a single long bar ( tropis) and a pair of lateral bars ( pastinum) ( Hart & Hart 1969, Fig. 1 View FIGURE 1 ; 1974, Fig. 10 View FIGURE 10 ). Each pastinum connects to the lateral framework of chitinous rods, from which the thoracic legs are suspended. Schulz (1976, Figs. 71, 78) illustrated a connecting rod in this position for Sclerochilus View in CoL sp. and Paradoxostoma View in CoL sp. ( Bythocytheridae View in CoL , Paradoxostomatidae View in CoL ). Tsukagoshi & Parker (2000, Fig. 10 View FIGURE 10 A) illustrated a U-shaped zygum for Callistocythere (Leptocytheridae) View in CoL . The sterinx, tropis and pastinum have not been seen in the Bairdiidae View in CoL studied.
Furca. The familiar term “furca” is used here without reference to any hypothesis about its segmental origin. Meisch (2007) showed that the “furcal rami” in Ostracoda may have been transformed from vibratory plates on the uropods (appendages of the posterior-most segment), whereas true furcae develop from the telson. He stated further (p. 197), “The transformation of a pair of vibratory plates into a pair of variously shaped structures adapted for locomotion and/or feeding demonstrates the powerful evolutionary potentiality of the epipodites of ostracodes.” In the case of Bairdioidea View in CoL , however, the furcae play no role in either feeding or locomotion. In resting position (in preserved specimens), the furcae wrap horizontally around the genitalia (hemipenes or genital lobes) (Figs. 9A–B, 17A, E). This suggests that the setae of the furcae (especially seta 2) may have a cleaning function. The disproportionate length of seta 2 (more than twice the length of any other seta, though not long enough to extend outside of the domicilium) further implies a special function.
The setal armature of the furca is distinctive for several genera within Bairdiidae View in CoL , though not sexually dimorphic. Bairdoppilata View in CoL has seven setae, all fairly long ( Fig. 7 View FIGURE 7 G, 10B, 11B, 15D, 17F). Neonesidea View in CoL , Aponesidea View in CoL , and Havanardia View in CoL have seven setae, of which setae 6 and 7 (most proximal) are somewhat shorter than the others. Paranesidea View in CoL and Triebelina View in CoL have six setae, of which the two most proximal setae are shorter than others (18C). Mydionobairdia View in CoL apparently has only five setae.
Seta 2 (counting from the distal end) is always much longer than the others, with a basal shaft that is as long as the other setae, followed by a long distal bristle. The proximal part of the bristle (near the shaft) may be sharply bent and perhaps flattened. The five or six most proximal barbs of the bristle may be noticeably larger than the others and might have taxonomic value ( Figs. 11 View FIGURE 11 J, 15D, 17B, H–I). The finer distal spinules are nearly invisible in small species and often omitted from drawings.
Hemipenis. The male copulatory apparatus of Bairdiidae View in CoL , where known, is thought to be distinctive for each species. General resemblances at the generic level are suspected but not yet reliably delineated. This complex appendage was described by Müller (1894, for Neonesidea View in CoL ) and Rome (1960, for G. c o ro n a t a), and Danielopol interpreted homologies of the parts (1972, for Neonesidea View in CoL ).
Each hemipenis is composed of three articulated parts, usually termed the basal, medial and terminal segments. In fact, the basal segment consists of two overlapping branches, joined posteriorly, which swing apart or can easily be torn apart as a V ( Fig. 10 View FIGURE 10 B). The dorsal branch of the basal segment is longer, oblong, and attached to the posterolateral edge of the zygum. The ventral branch of the basal segment is shorter, more or less quadrangular, and strengthened by a diagonal ridge, which runs from the posterior end of the dorsal branch to the dorsal edge of the median capsule ( Fig. 10 View FIGURE 10 E–F; 11B–D, F–G). Both branches house bundles of muscles. The medial segment (also known as the median capsule) displays several configurations in Bairdoppilata View in CoL , ranging from subcircular and complex to crescentic and streamlined. It is filled with two groups of outward-fanning muscles ( Figs. 10 View FIGURE 10 C–F, 11C–H). In the shallow-water species the distal end carries various conical, lamellar and hook-shaped prominences, of which the most ventral one may flare as a hood-like shield over the others in some species. The terminal segment is relatively small and lamelliform, ending in a ridged projection above an incised groove, which receives the copulatory tube ( Figs. 10 View FIGURE 10 C–F, 11C–H).
The sleeve of the copulatory tube in shallow-water species of Bairdoppilata View in CoL arises fairly close to the posterior end of the median capsule and arches gracefully through 180o to 360o ( Figs. 10 View FIGURE 10 C–F, 11C–H). It is held in place by distal latches but not constrained within a channel, as it is in Neonesidea View in CoL .
Some of the deep-water species of? Bairdoppilata View in CoL have a more linear, more conspicuously hinged hemipenis with a crescentic medial segment, few or no distal prominences, and a shorter, nearly straight copulatory tube ( Brandão 2008, Figs. 8 View FIGURE 8 M–Q, 9A, G).
Genital lobe. The external form of each genital lobe is a pendant ovoid ( Fig. 12 View FIGURE 12 H–I, 13H, 17C–D, F). A small copulatory opening leads through a nearly straight canal into the seminal receptacle. A larger orifice opens out of the oviducts. A spirally coiled canal connects the seminal receptacle with the latter orifice, and a set of muscles pumps sperm through the spiral canal for fertilization of the eggs ( Müller 1894). Müller (1894) considered the number of coils and thickness of the spiral canal to be useful for distinguishing some species.
The genital lobes have not been illustrated for many species of Bairdoppilata View in CoL . Their appearance changes with viewing aspect and focus, and their taxonomic potential remains undetermined. In the species examined here, they have subquadrate outlines with a flattened venter ( Fig. 12 View FIGURE 12 G–I, 13H, 17C–D, F, O). The internal tubes make relatively few coils and occupy only a small part of the volume.
In Paranesidea View in CoL sp. 1 and sp. 2 the genital lobes are more elongate, twisted in lateral outline and taper ventrally ( Figs. 20 View FIGURE 20 N–P, 21H–I). The coiled tubes are thicker, more complexly coiled, occupy more of the internal volume, and their internal surfaces are striated internally ( Figs. 20 View FIGURE 20 P, 21H–J). These internal, radially arranged, longitudinal striations might be sculptural lines or contractile fibers. This feature has not been observed in Neonesidea View in CoL or Bairdoppilata View in CoL .
Masticatory organ. All living Bairdiidae View in CoL have a unique masticatory organ (chewing structure) at the top of the esophagus ( Figs. 7 View FIGURE 7 F, 11I, 12J, 15C, 16C, 17J). It is often ignored but deserves more attention, and Maddocks (2013) suggested that it might have taxonomic significance. This structure is illustrated here for the four species of Bairdoppilata View in CoL , as it was for the five Hawaiian species of Neonesidea ( Maddocks 2013) View in CoL . Its function and taxonomic potential will be evaluated in a separate paper.
Median eye. The median eye of Bairdiidae View in CoL has been described chiefly for species of Neonesidea View in CoL ( Müller 1894, Tanaka 2005, Maddocks 2013). Müller stated that it is poorly developed in other species-groups. The four species of Bairdoppilata View in CoL investigated here all have medium-sized to large, red-brown eyes, somewhat more conspicuous than those of Neonesidea View in CoL . The median eye is situated below the carapace on the dorsal midline of the body, at the anterior edge of the isthmus, just behind the base of the antennules and in front of the midgut ( Figs. 10 View FIGURE 10 A, 12L, 13C–E). In lateral view, if the anterior food ball is large, the eye may be obscured. In B. scaura View in CoL the eye is especially large and dark-colored, with long, cone-shaped lateral cups ( Figs. 10 View FIGURE 10 A, J). In the species of Paranesidea View in CoL examined here the eye is somewhat smaller and lighter in color than in Bairdoppilata View in CoL ( Figs. 20 View FIGURE 20 D, K).
In the shallow-water species of Bairdoppilata View in CoL reviewed here, there is no eye-spot or ocular lens embedded in the carapace, as described for the Paleocene-Eocene genus Oculobairdoppilata View in CoL by Itterbeeck et al. (2007). They reviewed reports of fossil bairdiids with eye-spots and noted that such species occur lower in the photic zone, below an estimated depth of 50– 175 m. None have been reported living.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Bairdioidea |
|
Family |
|
|
Genus |
Bairdoppilata Coryell, Sample and Jennings, 1935
| Maddocks, Rosalie F. 2015 |
N. plumulosa
| Maddocks 2013 |
Neonesidea holdeni
| Maddocks 2013 |
Neonesidea (
| Maddocks 2013 |
Neonesidea (
| Maddocks 2013 |
Neonesidea michaelseni
| Hartmann 1984 |
Bairdoppilata geelongensis
| Hartmann 1980 |
Bairdoppilata sinusaquilensis
| Hartmann 1979 |
Bairdoppilata balihaiensis
| Hartmann 1978 |
Bairdoppilata portsamsonensis
| Hartmann 1978 |
Bairdoppilata angolensis
| Hartmann 1974 |
Bairdoppilata cytheraeformis
| Hartmann 1974 |
Bairdoppilata mocamedesensis
| Hartmann 1974 |
Bairdoppilata (Bairdoppilata) alcyonicola
| Maddocks 1969 |
Bairdoppilata (Bairdoppilata) cratericola
| Maddocks 1969 |
Bairdia simuvillosa
| Swain 1967 |
Neonesidea schulzi (
| Hartmann 1964 |
Bairdoppilata carinata
| Kornicker 1961 |
Nesidea cushmani
| Tressler 1949 |
Nesidea labiata Müller, 1908
| Muller 1908 |
N. tenera
| Brady 1886 |
Bairdia simplex
| Brady 1880 |
Bairdia hirsuta
| Brady 1880 |
Bairdia villosa
| Brady 1880 |
Bairdia coronata
| Brady 1870 |